State-of-the-Art Analytical Capability
Contact Us
- Group Leader
- Peter Stark
- Deputy Group Leader
- Dan Kelly
- Group Office
- (505) 667-5740
Unique Capabilities to support National Interests
C-CDE is home to unique experimental capabilities, spanning the range from advanced surface science, to polymer compounding and 3D printing, to niche analytical instrumentation supporting efforts in weapons materials, threat reduction, applied energy, public health, and the environment. We maintain close interaction between expertise in analytical chemistry of materials with our R&D programs in manufacturing, surveillance, and hazard response. This allows us to solve practical problems of national interest.
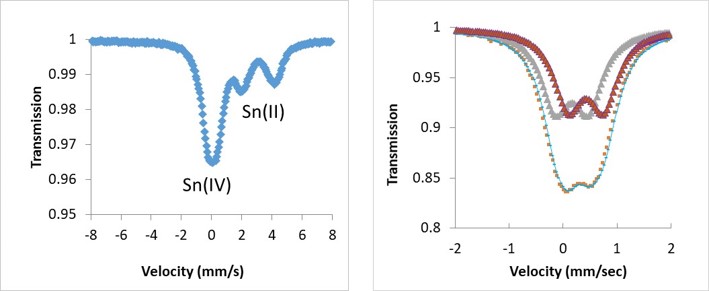
 Mössbauer spectroscopy is based on the Mössbauer effect and consists of recoil-free, resonant absorption and emission of gamma rays in solids. It is a versatile technique used to probe the chemical environment of an element such as oxidation state, magnetic environment, ligand effects, etc. More specifically, the technique uses a combination of the Mössbauer effect and Doppler shifts to probe the hyperfine transitions between the excited and ground states of the nucleus. Many isotopes exhibit Mössbauer characteristics such as 99Ru, 119Sn, 151Eu, 155Gd, 193Ir, 195Pt, but the most commonly studied isotope is 57Fe. Typically, three types of nuclear interactions may be observed: an isomeric shift, also known as a chemical shift; quadrupole splitting; and magnetic or hyperfine splitting. In the resulting spectrum, the gamma ray intensity is plotted as a function of the source velocity. At velocities corresponding to the resonant energy levels of the sample, a fraction of the gamma rays are absorbed, resulting in a drop in the measured intensity and a corresponding dip in the spectrum. Examples of the use of Mössbauer to provide information about the chemical environment of 119Sn and 57Fe absorbing nuclei are shown in the Figure below. This new capability will be soon installed at TA59, building 1.
Mössbauer spectroscopy is based on the Mössbauer effect and consists of recoil-free, resonant absorption and emission of gamma rays in solids. It is a versatile technique used to probe the chemical environment of an element such as oxidation state, magnetic environment, ligand effects, etc. More specifically, the technique uses a combination of the Mössbauer effect and Doppler shifts to probe the hyperfine transitions between the excited and ground states of the nucleus. Many isotopes exhibit Mössbauer characteristics such as 99Ru, 119Sn, 151Eu, 155Gd, 193Ir, 195Pt, but the most commonly studied isotope is 57Fe. Typically, three types of nuclear interactions may be observed: an isomeric shift, also known as a chemical shift; quadrupole splitting; and magnetic or hyperfine splitting. In the resulting spectrum, the gamma ray intensity is plotted as a function of the source velocity. At velocities corresponding to the resonant energy levels of the sample, a fraction of the gamma rays are absorbed, resulting in a drop in the measured intensity and a corresponding dip in the spectrum. Examples of the use of Mössbauer to provide information about the chemical environment of 119Sn and 57Fe absorbing nuclei are shown in the Figure below. This new capability will be soon installed at TA59, building 1.

X-Ray Photoelectron Spectroscopy (XPS) is a surface science technique used to measure a materials element composition and chemical/electronic state of atoms in a material. A recent breakthrough in XPS technology is the operation of the system at ambient pressure, enabling the study of materials in real-world conditions. This capability supports research in areas of corrosion, materials aging, fuel cells, and nuclear fuel cycle.
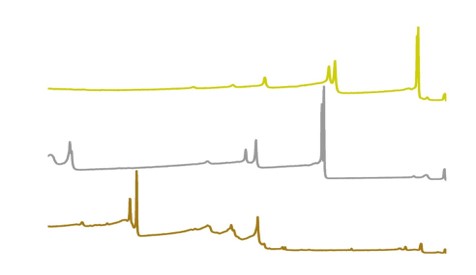
CDE is routinely asked to analyze uranium metal, uranium oxide, uranium hydride, and uranium nitride for chemical identification, isotopic composition, sample homogeneity, and purity. We have several different methods to characterize uranium, including laser induced breakdown spectroscopy (LIBS), laser ablation inductively coupled mass spectrometry (LA-ICP-MS), and solution ICP-MS.
Chemical Identification

Photo courtesy of SciAPS
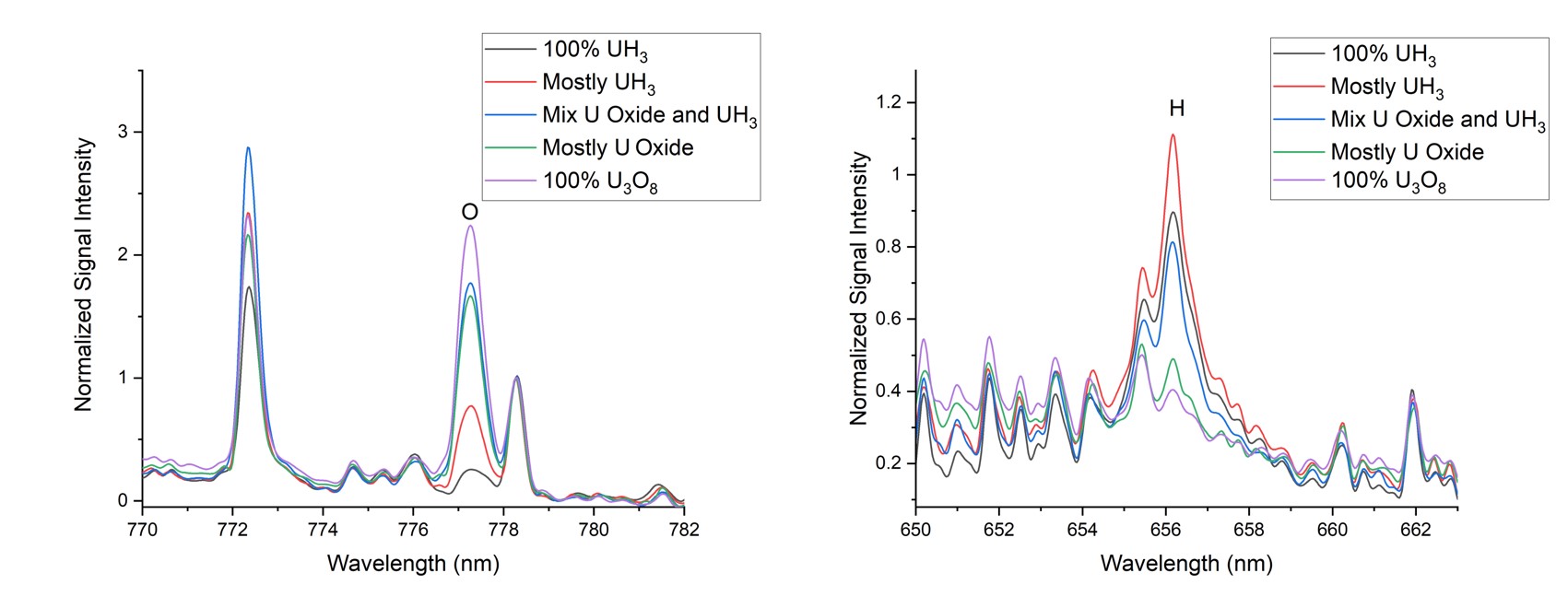
LIBS is a rapid, optical spectroscopy technique that uses a focused pulsed laser to ablate nanograms of material. The material is vaporized, ionized, and then emits light at characteristic wavelengths of the material sampled. With a commercial off the shelf handheld LIBS system from SciAps, we have developed a method to determine if uranium corrosion is uranium hydride or uranium oxide. The application program is on the HH LIBS and reports a result following the rapid analysis (seconds).
Sample Homogeneity

New Wave Research (NWR) 213
Laser Ablation System
Photo courtesy of ESI
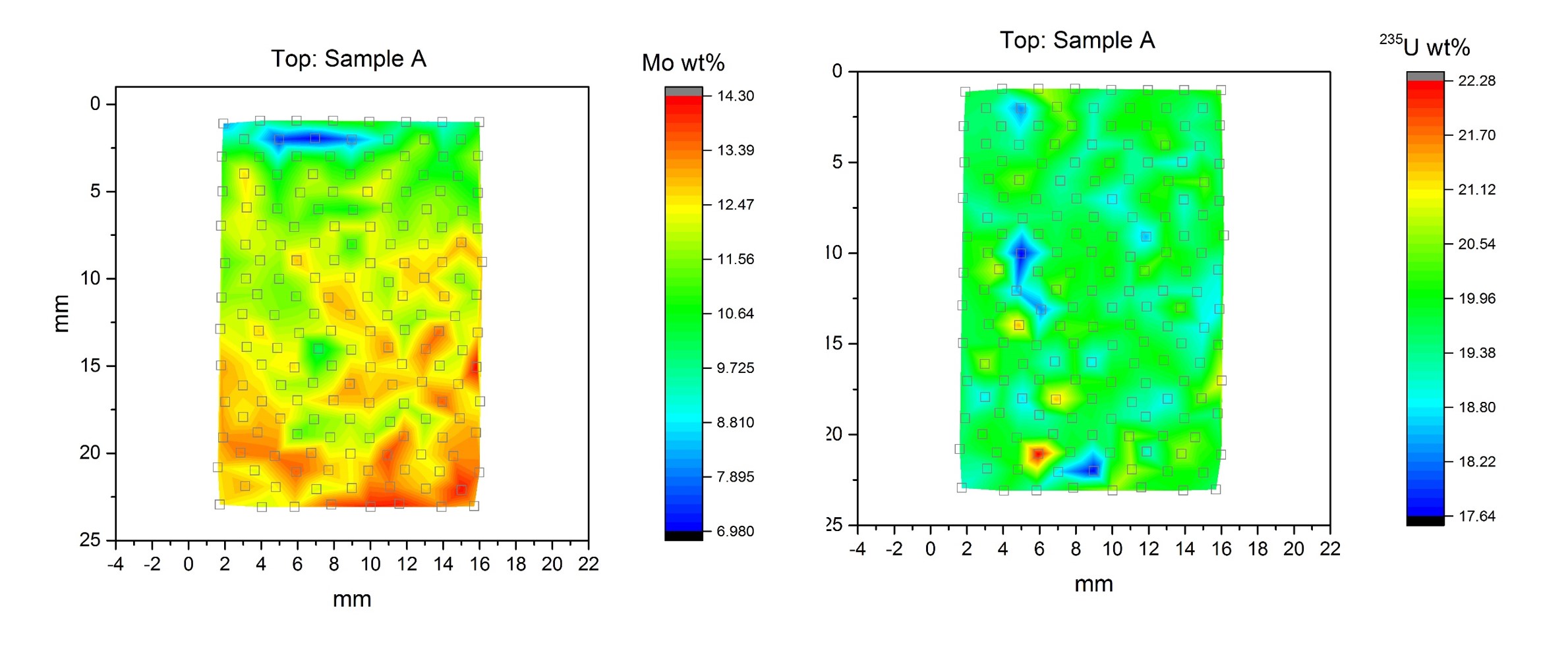
LA-ICP-MS is a robust analytical technique that requires little to no sample preparation allowing for high throughput. A sample is analyzed directly allowing for spatial information to be maintained (mapping capability). The ablated material is transferred from the sample chamber via a carrier gas to the ICP-MS for ionization and detection. The technique is mostly used for trace metal analysis (ppm – low wt% level) but bulk analysis can be done with appropriate standards. Analysis of a uranium – molybdenum alloy is shown below where the homogeneity of Mo and 235U can be seen.
Sample Purity and Isotopic Composition

Photo courtesy of Thermo Scientific
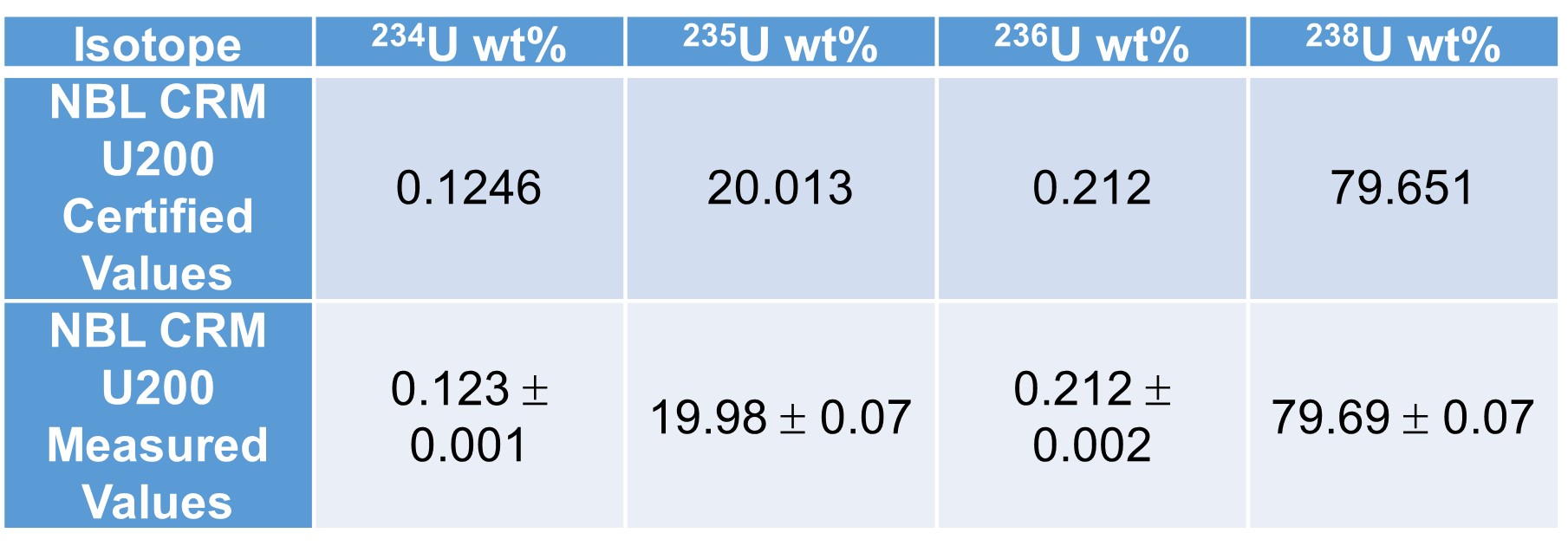
CDE is often asked to analyze the purity of metals and oxides, including uranium. Isotopic composition is also important when analyzing uranium samples. Solution phase ICP-MS is a robust analytical instrument that uses an argon plasma to ionize a sample. The triple quad iCAP Q separates the elements based on m/z. Certified elemental standards and isotopic standards are used for quantification and in calculating the isotopic composition of the sample. In addition, UTEVA columns are typically utilized to separate uranium from the impurities in the sample to achieve lower limits of detection.

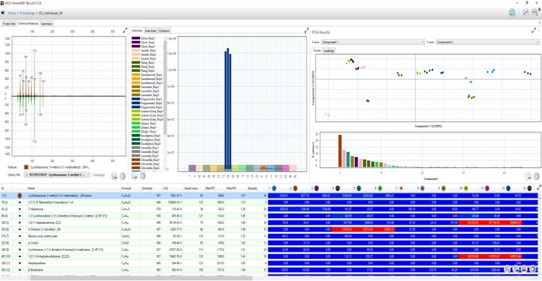
This state-of-the-art 4-dimensional gas chromatography / mass spectrometry instrument, GCxGC High-Resolution Time-of-Flight Mass Spectrometer enables new methods for analyzing complex samples for organic constituents. The instrument supports R&D in analysis of polychlorinated biphenyl (PCB) contamination, inorganic polymers, biopolymers, and bacterial metabolites.