Phosphorus-Containing Ligands Support f-Block Complexes
- Stephanie H. Carpenter
Ligand design and synthesis
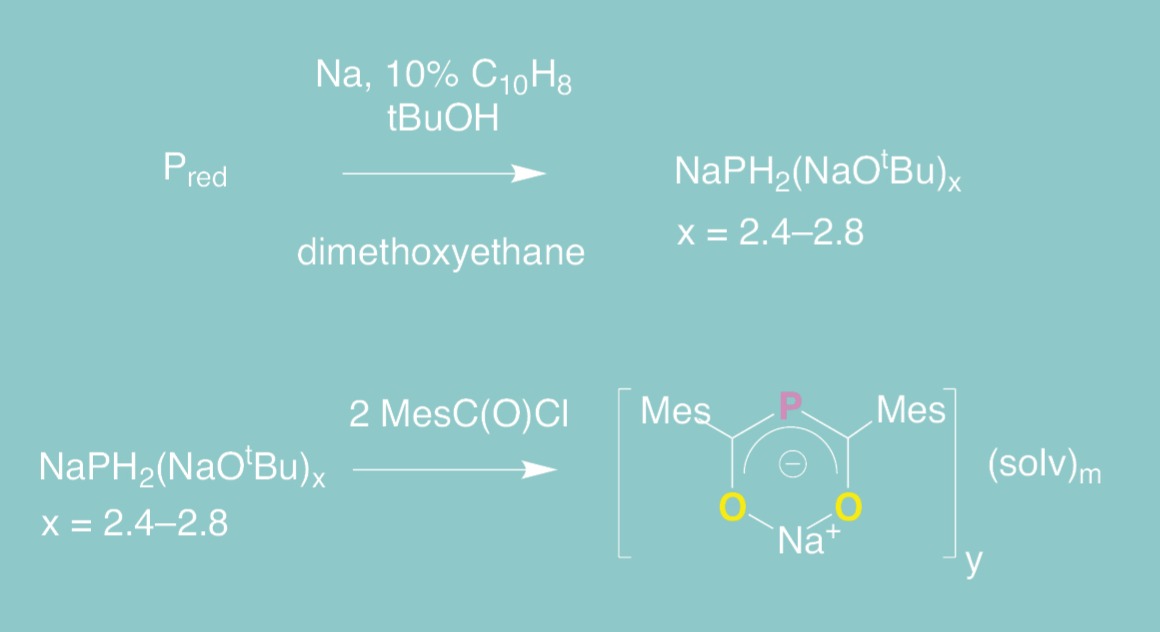
Using metal series to investigate bonding trends
The radiological concerns and technical challenges of performing research with transuranic elements require rigorous studies with surrogate metals to fully understand the molecular system. Lanthanide metals are often employed as surrogates for the more radioactive transuranic elements given their similar size and reactivity, and as a consequence, the more rigorous and less hazardous lanthanide studies take far longer than analogous, more selective actinide investigations. Our fundamental study into the bonding interactions of actinide BAP complexes therefore began with the lanthanide metals. We hypothesized europium (Eu) and erbium (Er) would provide access to unusual reactivity and magnetic properties while simultaneously serving as surrogates for actinide metals.
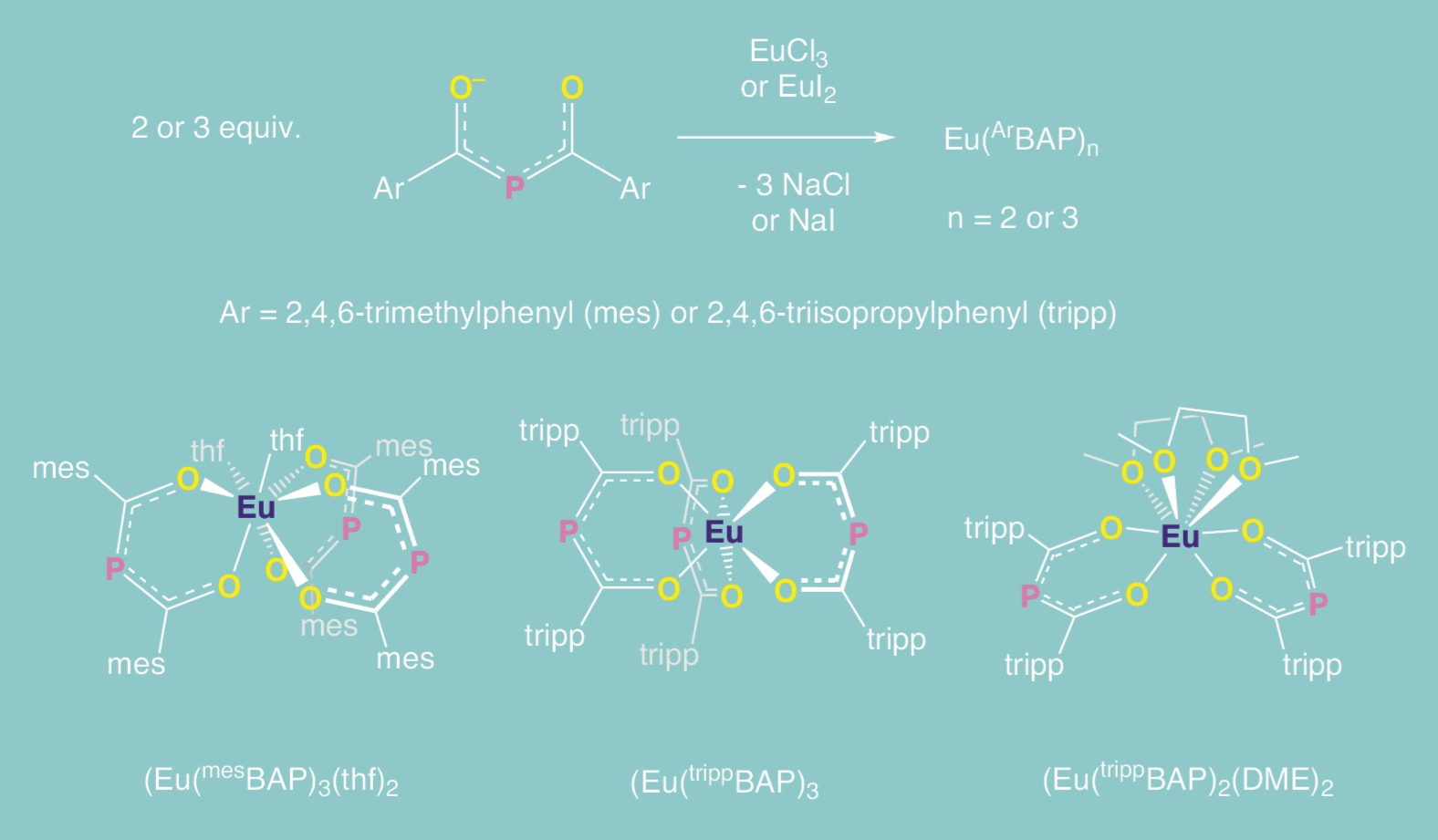
Initial europium studies showed the feasibility of using the BAP motif to stabilize molecular lanthanide complexes over multiple oxidation states. Encouraged by these results, further investigations into the reactivity of the trippBAP moiety were performed with erbium, a lanthanide metal with a spin-active nucleus that provides an additional spectroscopic handle for characterizing and understanding the electronic structures of these compounds. After successfully synthesizing an erbium(III) complex with three ligands, Er(trippBAP)3, reduction by one or two electrons allowed for the formation of new complexes in the formal oxidation states of erbium(II) and (I) (Fig. 3). Magnetic studies to confirm these oxidation states are ongoing and it is likely that these formally designated oxidation states do not capture their true, complex electronic structures. Nevertheless, the electronic flexibility imparted by the ligand through the phosphorus atom in the backbone may provide electronic stabilization of formal oxidation states that are unprecedented for erbium.
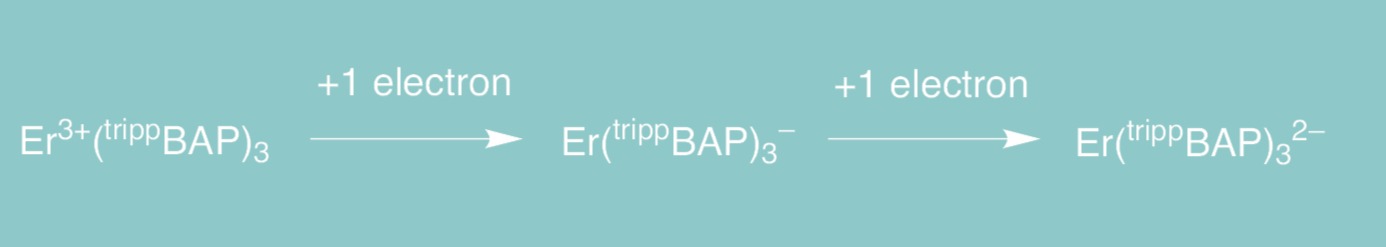
Transuranic elements are only available in milligram quantities and working on these scales often provides challenges in the laboratory. In anticipation of these challenges, we attempted to prepare europium- and erbium-BAP complexes on similar scales, and were successful using approximately 5 mg of metal content of europium and erbium starting materials. These encouraging results supported the viability of our proposed reactivity studies with BAP ligands and uranium and the transuranic elements.
Molecular uranium BAP complexes
The successful preparation of both the europium and erbium BAP series on a small scale allowed us to adapt this synthesis to uranium. We hypothesized the addition of the mesBAP ligand to uranium(III) iodide in a 3:1 ratio would result in the formation of a uranium(III) complex, which would be ideal for electronic structure studies via electron paramagnetic resonance (EPR) spectroscopy. Unfortunately, uranium is prone to single electron chemistry and ligand rearrangement, often resulting in the formation of unexpected compounds. As such, this reactivity led to the isolation of a uranium(IV) complex with four ligands, U(mesBAP)4, which was consistently and reproducibly isolated with no observed formation of the desired uranium(III) complex, despite the 3:1 stoichiometry of ligand to metal.
The mesityl groups on the mesBAP ligands are directed away from the large, reactive uranium center (Fig. 4), providing little steric protection and allowing for the unwanted formation of a uranium(IV) complex from uranium(III) starting materials. To mitigate undesired ligand rearrangement and disproportionation reactivity, we reasoned that the increased steric bulk in trippBAP would limit the number of ligands bound to the uranium center and stabilize the desired uranium(III) complex. The analogous reaction using trippBAP (with a 3:1 ratio of ligand to uranium) successfully yielded the formally trivalent uranium complex, U(trippBAP)3.
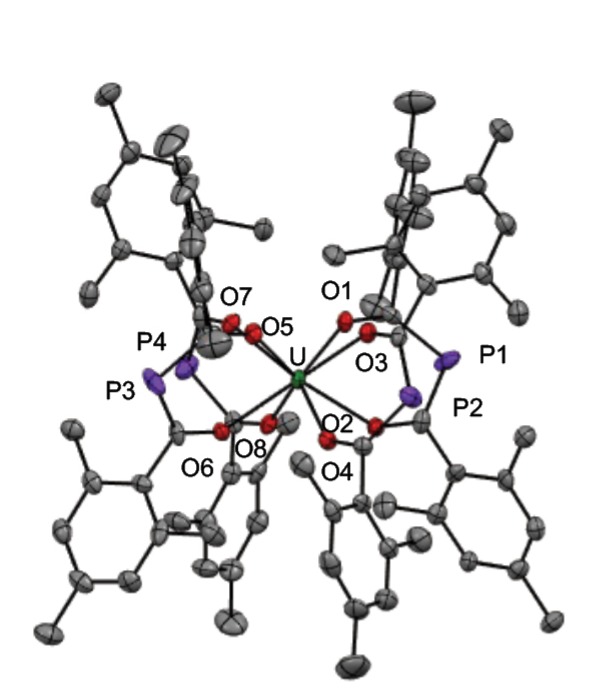
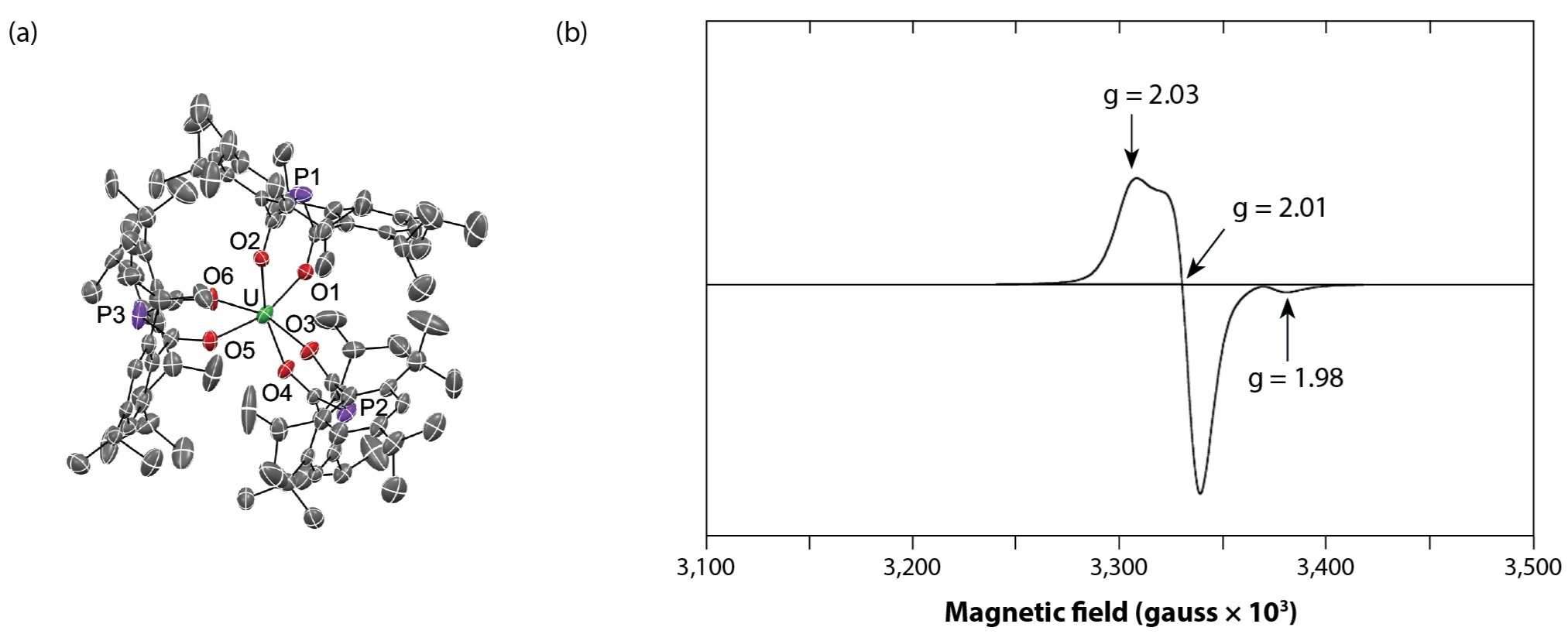
Electronic structure studies employing a combination of single-crystal X-ray diffraction, EPR, nuclear magnetic resonance, and UV/vis./near-infrared spectroscopies suggest a complicated electronic structure for U(trippBAP)3 beyond the expected simple uranium(III) ion. Solid-state metrical parameters suggest trippBAP may be able to accept a significant amount of electron density, whereas EPR studies of U(trippBAP)3 reveal a signal that does not significantly shift from the spin-only value of a free electron (Fig. 5). This observation suggests U(trippBAP)3 has an unpaired electron in a molecular orbital containing both metal and ligand character, where the uranium center appears more as a uranium(IV) ion with an electron delocalized on the ligand. The disappearance of the EPR signal at room temperature further supports this observation. Rather than assuming electron placement on the metal or on the ligand, we feel the electronic structure studies of U(trippBAP)3 suggest the uranium center exists on a continuum between the (III) and (IV) oxidation states.
Transitions into transuranic elements
Because both the lanthanide and uranium BAP complexes were accessible on a small scale (approximately 5 mg metal), we were encouraged that this chemistry could be adapted to the transuranic elements, specifically neptunium (Np) and plutonium (Pu). Neptunium is the most accessible transuranic metal and has unique reactivity—arising from its electronic properties—that remains poorly understood compared to uranium. Plutonium, meanwhile, exists as an outlier in the transuranic series studied, as it retains a high affinity for oxygen coordination yet is less prone to spontaneous oxidation to the tetravalent oxidation state compared to uranium and neptunium. By comparing homologous reactivity between these metals with mesBAP, we could directly contrast the effect of these elemental traits on the outcomes of reaction products. For mesBAP chemistry in particular, a motivation for the work was the hope of observing an outlier in the reaction series using plutonium starting materials. Fundamental reactivity investigations involving uranium, neptunium, and plutonium are rare, but Los Alamos is primed to lead the field of molecular actinide chemistry by encouraging these types of studies.
The neptunium BAP study examined the reactivity of mesBAP with a 3:1 ratio of ligand to neptunium starting materials in a reaction designed to closely mimic the conditions used for U(mesBAP)4. The product was identified as Np(mesBAP)4 using single-crystal X-ray diffraction, showing homologous reactivity between uranium and neptunium, where an unanticipated single electron event occurs and the product arises from spontaneous oxidation. The isolation of a neptunium(IV) complex shows the adaptability of this ligand set towards transuranic elements and highlights some of the challenges when attempting to design ligands and reactivity specific to individual actinides. Current studies focus on exploring the electronic structure of Np(mesBAP)4 using EPR spectroscopy, with initial analysis suggesting that the unpaired electrons, as expected, are found localized around the metal center.
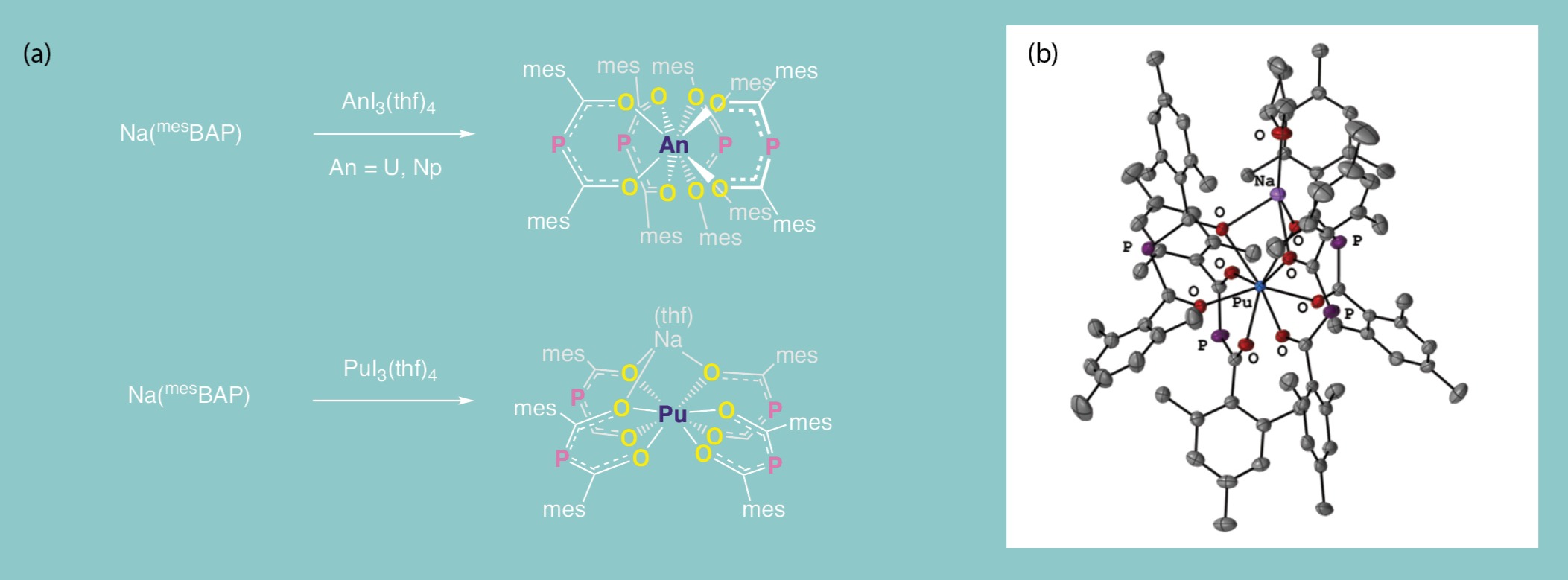
The addition of mesBAP in a 3:1 ratio of ligand to plutonium starting material, homologous to the uranium and neptunium reaction conditions, gave rise to a unique product. Four mesBAP ligands were observed around the plutonium center, but a sodium atom from the ligand was found bound to the ligand oxygen atoms, leading to the assignment of a plutonium(III) oxidation state (Fig. 6). This result highlights the unique properties of plutonium, compared to uranium and neptunium, that give rise to behavior distinct from other actinide metals. Our approach to synthesis in this investigation is also important: by performing reactions in a series using related elements, ligands, and oxidation states, a stronger understanding of nuanced chemical behavior can emerge.
Summary
Bis(acyl)phosphide (BAP) ligands show unique reactivity towards uranium and neptunium, resulting in unexpected electronic structure assignments of the first isolated actinide BAP complexes. Spectroscopic investigations suggest the trippBAP moiety can accept an appreciable amount of electron density that raises questions over accompanying metal oxidation states. The combined reactivity and magnetism studies of BAP ligands with europium, erbium, and uranium provide the foundation and context of this system while pushing the chemistry forward to neptunium and other transuranic elements. This knowledge can be used for the continued development of waste remediation, metal recycling efforts, and furthering our understanding of the chemical reactivity of plutonium.
Acknowledgments
This work is a collaborative effort with several other researchers: Nikki J. Wolford, Jesse Murillo, Ju Chen, Brennan S. Billow, Taylor V. Fetrow, Nathalia Cajiao, Joshua Mengell, Michael T. Janicke, Andrew J. Gaunt, Michael L. Neidig, Martin L. Kirk, and Aaron M. Tondreau were all critical to the progress and understanding of this project. Financial support was provided by the U.S. Department of Energy through the Office of Basic Energy Science and the Heavy Element Chemistry program (HEC@ LANL, 2020LANLE372, DE-AC52-06NA25396) and the Seaborg Institute for the postdoctoral fellowship of Stephanie H. Carpenter.
Stephanie Carpenter
Stephanie joined LANL in November 2019 under the mentorship of Aaron M. Tondreau and Ping Yang. She was a Seaborg Institute Postdoctoral Fellow from August 2020 to June 2022. Stephanie is interested in the synthesis of molecular actinide and transition metal complexes and understanding the complex electronic structure of these complexes via numerous spectroscopic techniques. She is currently a scientist in the Stockpile and Enterprise Analytics Office, focusing on data analysis for enterprise-wide problems.
Further reading:
- S.H. Carpenter, N.J. Wolford, B.S. Billow, T.V. Fetrow, N. Cajiao, A. Radović, M.T. Janicke, M.L. Neidig, A.M. Tondreau, “Homoleptic Uranium-Bis(acyl)phosphide Complexes,” Inorg. Chem., 2022, 61, 32, 12508.
- J. Chen, S.H. Carpenter, T.V. Fetrow, J. Mengell, M.L. Kirk, A.M. Tondreau, “Magnetism Studies of Bis(acyl) phosphide-Supported Eu3+ and Eu2+ Complexes,” Inorg. Chem. 2022, 61, 46, 18466.
- A. Huber, A. Kuschel, T. Ott, G. Santiso-Quinones, D. Stein, J. Bräuer, R. Kissner, F. Krumeich, H, Schönberg, J. Levalois-Grützmacher, H. Grützmacher, “Phosphorus-Functionalized Bis(acyl)phosphane Oxides for Surface Modification,” Angew. Chem. Int. Ed. 2012, 51, 4648.
- A. Eibel, M. Schmallegger, M. Zalibera, A. Huber, Y. Bürkl, H. Grützmacher, G. Gescheidt, “Extending the Scope of Bis(acyl)phosphane Oxides: Additional Derivatives,” Eur. J. Inorg. Chem., 2017, 2017, 2469.
- D.S. Michael and G. Schreckenbach, “Bis(acyl)phosphide Complexes of U(III)/U(IV): A Case of a Hidden Redox-Active Ligand,” Inorg. Chem., 2024, 63, 21, 9711-9714.
- S.H. Carpenter, J. Mengell, J. Chen, M.R. Jones, M.L. Kirk, A.M. Tondreau, “Determining the Effects of Zero-Field Splitting and Magnetic Exchange in Dimeric Europium(II) Complexes,” Inorg. Chem., 2024, 63, 19, 8516-8520.