Probing the Effect of Hydrogen on Uranium
- Mary O'Brien

Steel pipelines used to transport oil and gas are exposed to high hydrogen concentrations in the form of hydrogen sulfide gas, which has led to numerous instances of pipeline leaking or rupture through hydrogen embrittlement. In uranium, even small amounts of hydrogen picked up during manufacturing processes like welding is enough to cause an unexpected reduction in ductility.

Hydrogen embrittlement is a long-standing challenge in metallurgy—early onset failures can occur in metallic systems after they are exposed to hydrogen, whether it is during manufacturing or while in service. For example, steel pipelines used to transport oil and gas are exposed to high hydrogen concentrations in the form of hydrogen sulfide gas, which has led to numerous instances of pipeline leaking or rupture. In uranium, even small amounts of hydrogen picked up during manufacturing processes like welding or salt-bath annealing are enough to cause an unexpected reduction in ductility. Reduced ductility early in manufacturing can result in unexpected cracking during downstream manufacturing processes like drawing. Furthermore, when metallic uranium is used in nuclear applications such as fuels, it can be exposed to hydrogenous environments (e.g., aqueous and acidic solutions), both in the reactor and during end-of-life storage. As one of the few institutions in the world entrusted with actinide research, Los Alamos National Laboratory has a duty to illuminate the failure mechanisms of uranium materials for both global security needs and nuclear power.
“Uranium affords a unique opportunity to study the effect of hydrogen on the deformation of metals ”
Hydrogen deformation mechanisms are elusive
The combination of strength and ductility required in many common engineering applications such as steel pipelines or cars is supplied largely from the high symmetry of the metallic crystal structures of such materials (Fig. 1a). Metals accommodate deformation largely by movement of defects, also known as slip, along a specific direction and plane, the combination of which is known as a slip system. Scientists and engineers have extensively studied the impact of hydrogen on these high-symmetry crystal structures, but despite a century of research, the mechanisms by which hydrogen causes early-onset failures are not well understood. This is partly because high-symmetry crystals have many symmetrically equivalent slip systems, which results in complex defect interactions that are difficult to decipher. Many engineering metals (nickel, aluminum, some stainless steels, etc.) have a face-centered cubic structure, containing two planes of atoms and six directions along which defects can move (Fig. 1a). The complex interactions that arise when defects interact along these planes and directions convolutes the interpretation of the effect of hydrogen on any individual mechanism.
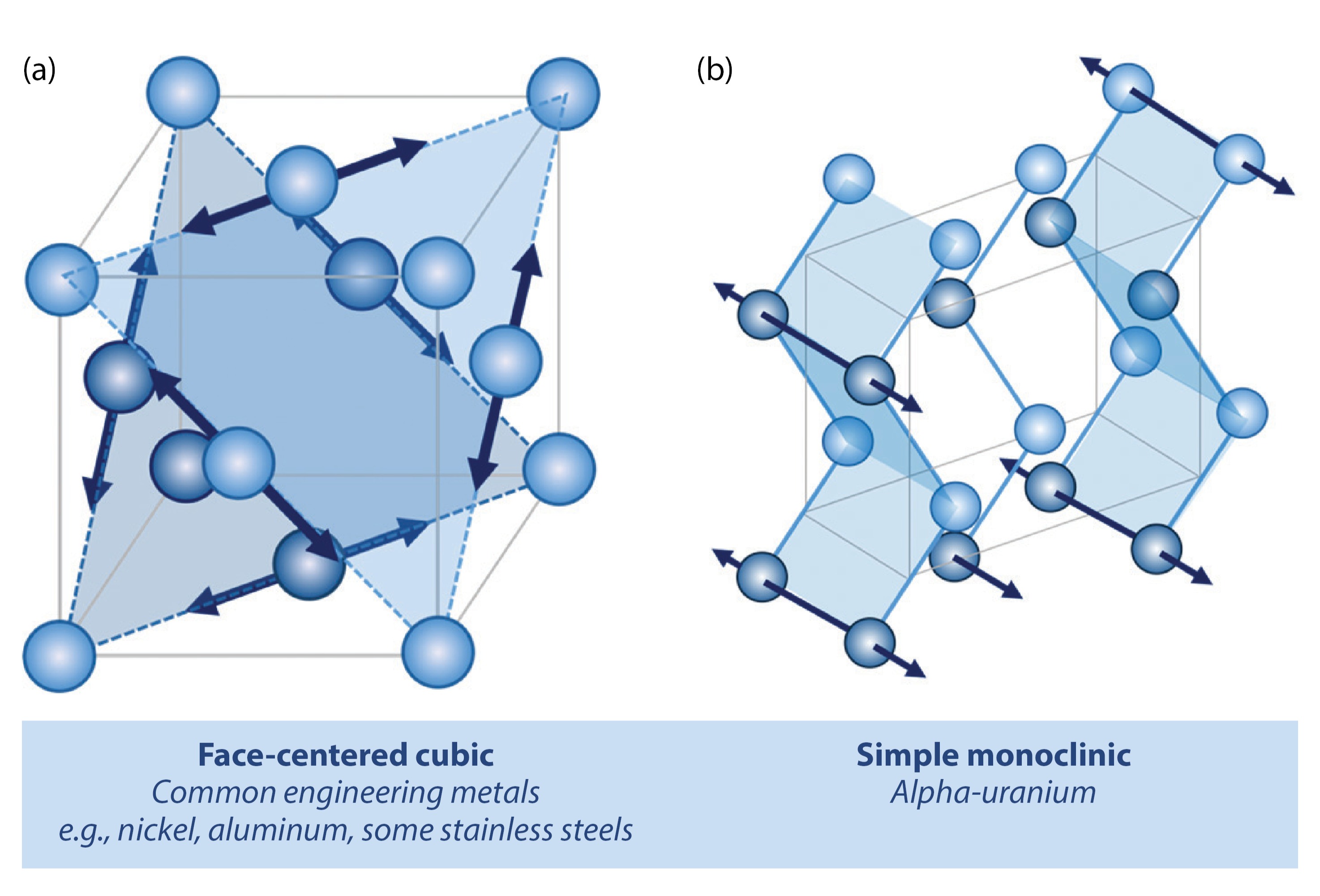
Alpha-uranium, with its low-symmetry, orthorhombic crystal structure, provides a unique opportunity to assess hydrogen effects on deformation. Atoms in alpha-uranium are arranged in a corrugated structure (Fig. 1b), which has far fewer slip planes to accommodate deformation. As a result, alpha-uranium offers a unique opportunity to deconvolute the effect of hydrogen on deformation, providing insight into both uranium deformation and the broader mechanisms of hydrogen effects in metals.
Understanding hydrogen/uranium interactions is not without its own challenges. Hydrogen has an exceptionally low solubility in uranium at room temperature (~0.2 wppm). Upon saturation, it forms uranium hydride, which results in an approximate 70% volume expansion, posing significant safety problems as it is pyrophoric (spontaneously combusts in oxygen). Due to the low solubility and high affinity for hydrogen, and accompanying safety issues, the study of hydrogen embrittlement in uranium has largely focused on understanding the initiation and growth of uranium hydride. However, many researchers have observed that the addition of hydrogen can still drastically reduce the ductility of uranium even at concentrations below the solubility limit.
Basic aspects of hydrogen embrittlement
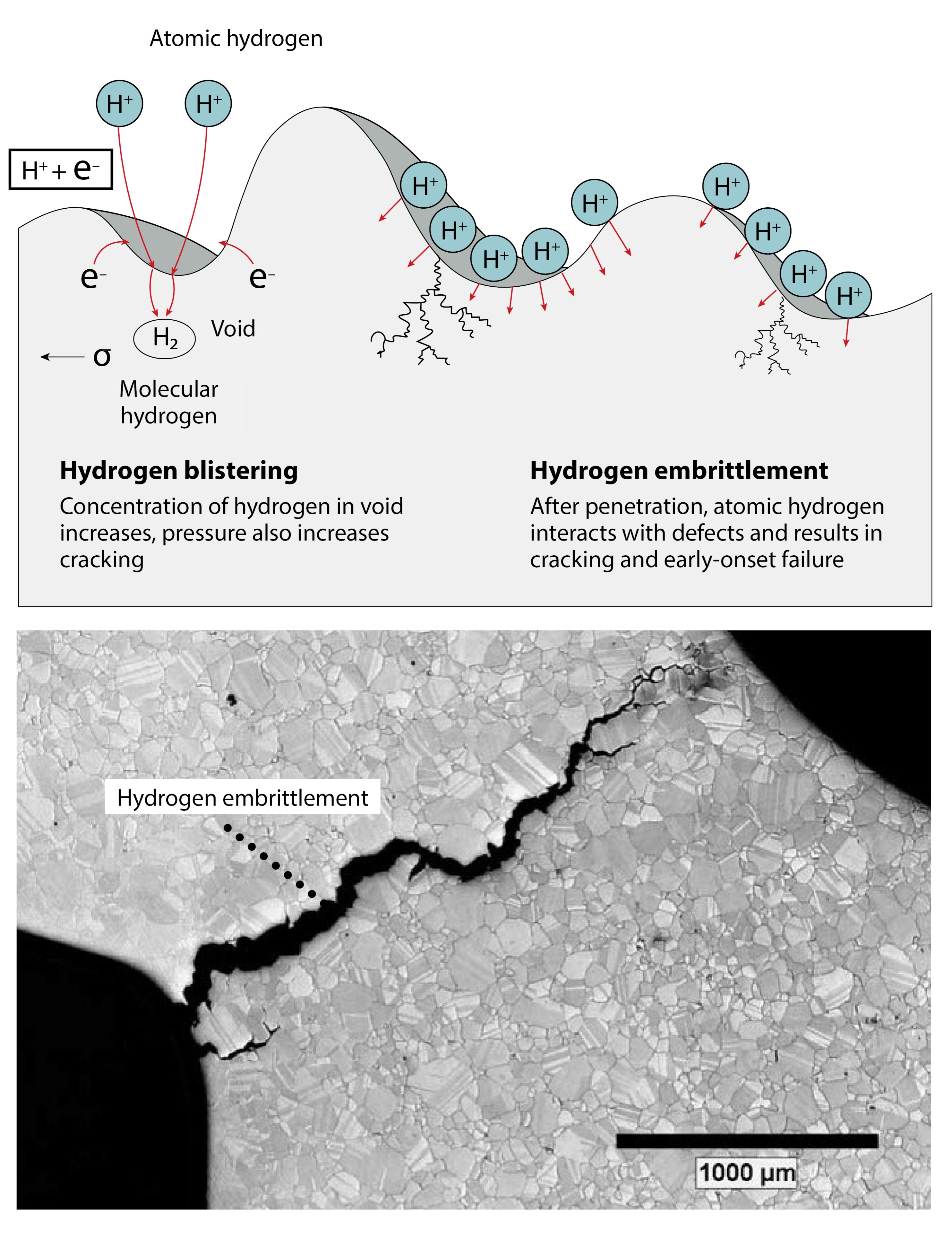
Researchers have identified several different mechanisms to explain reductions in ductility from hydrogen, however, most relevant to our studies is the hydrogen-enhanced localized plasticity (HELP) theory. This theory proposes that hydrogen affects defects, both in the way individual defects move and the larger structures that the accumulation of defects create. Plasticity results when a material permanently deforms—this permanent deformation starts with an internal defect movement, and accumulation of these defects can result in material failure. The defects relevant to this theory are line defects in the crystal, which are termed dislocations.
Supporting this theory, researchers have observed that hydrogen reduces the repulsive force between two defects, which results in higher defect densities in samples deformed in the presence of hydrogen. When too many defects accumulate locally, internal void or crack formation occurs. Thus, because more dense accumulations of defects occur in the presence of hydrogen than would occur in its absence, hydrogen can cause early onset failure. Historically, research has focused on studying these dense defect structures after fracture has occurred. However, more work is needed to understand the effect of hydrogen on initial defect movement before fracture occurs. This work explores the latter, examining the hypothesis that hydrogen reduces the amount of energy required to initiate movement of line defects.
The low-symmetry crystal structure of alpha-uranium poses both opportunities and challenges for observing the effect of hydrogen on initial defect movement. As described above, its low symmetry has fewer symmetrically equivalent slip systems, simplifying the deformation mechanism. However, its more complex macrostructure creates some difficulties. Within a larger piece of metal, there are groupings of similarly oriented crystals, referred to as grains. The distribution of orientations of these grains is known as crystallographic texture, which can be drastically affected by the manufacturing process. This is important for alpha-uranium because it results in deformation processes that are highly dependent on the orientation of the crystal, known as anisotropy.
Effects of processing on properties of uranium
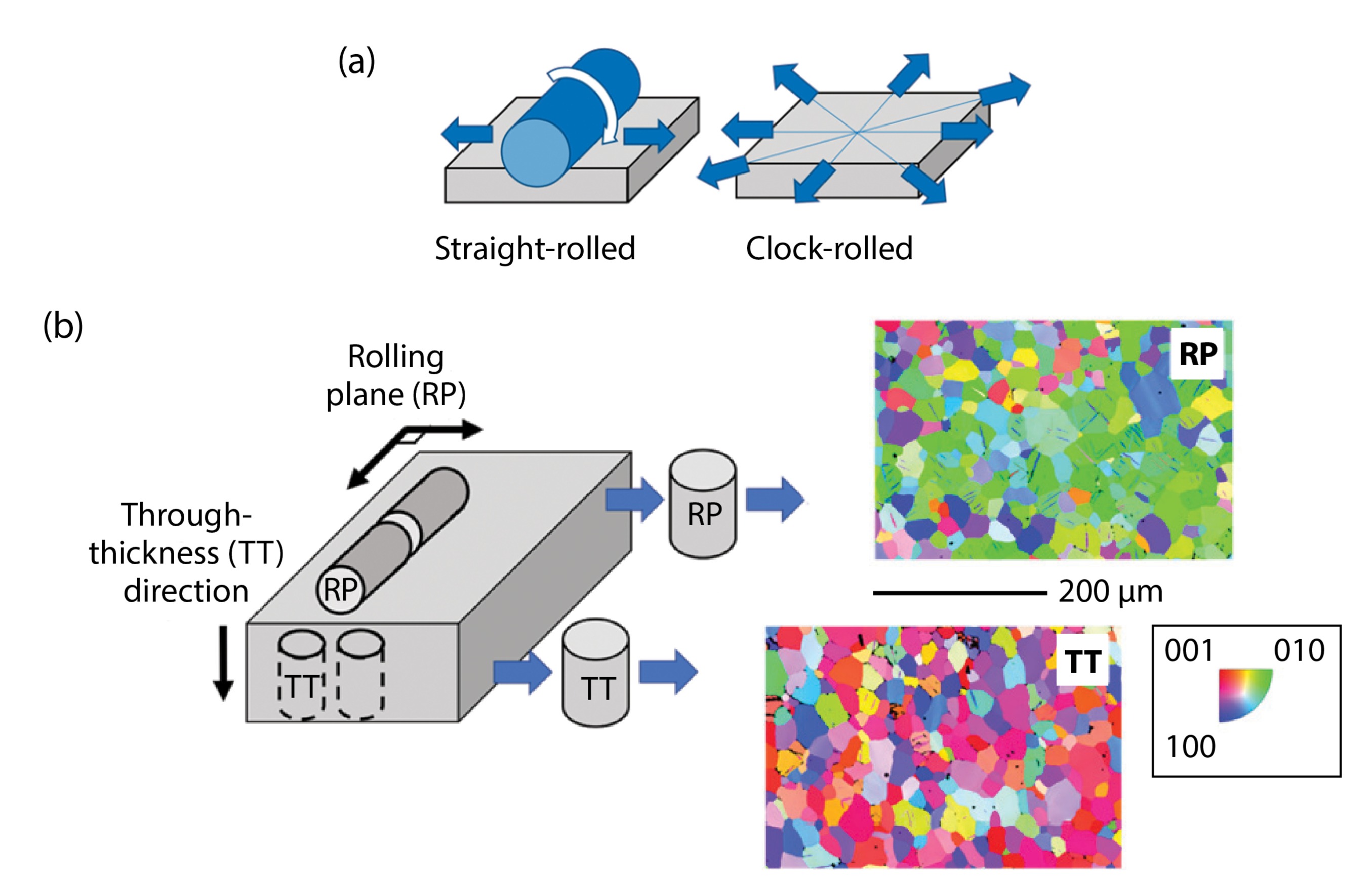
Because of anisotropy in uranium, the traditional rolling process used with most metals (straight-rolling) has been modified by metallurgists to achieve more homogeneous properties (Fig. 3a). This more complicated process, called clock-rolling, creates a much more homogeneous texture within the rolling plane. However, a different texture persists between the through-thickness direction and the rolling plane, which is seen in the electron backscatter diffraction (EBSD) inverse pole figure (IPF) micrographs for both of these directions (Fig. 3b).
A result of the differences between the rolling plane and through-thickness textures is that deformation is accommodated by different mechanisms depending on how a testing specimen is machined from a plate. In a through-thickness sample (Fig. 3a), the stress is largely accommodated by movement of line defects (i.e., dislocations). In contrast, in the rolling plane direction, stress is accommodated by a mechanism called twinning (details of which can be found elsewhere but are not the focus of the current work). Because we seek to understand the effect of hydrogen on the onset of line defect movement, discussion of results will be limited to the through-thickness textured specimens.
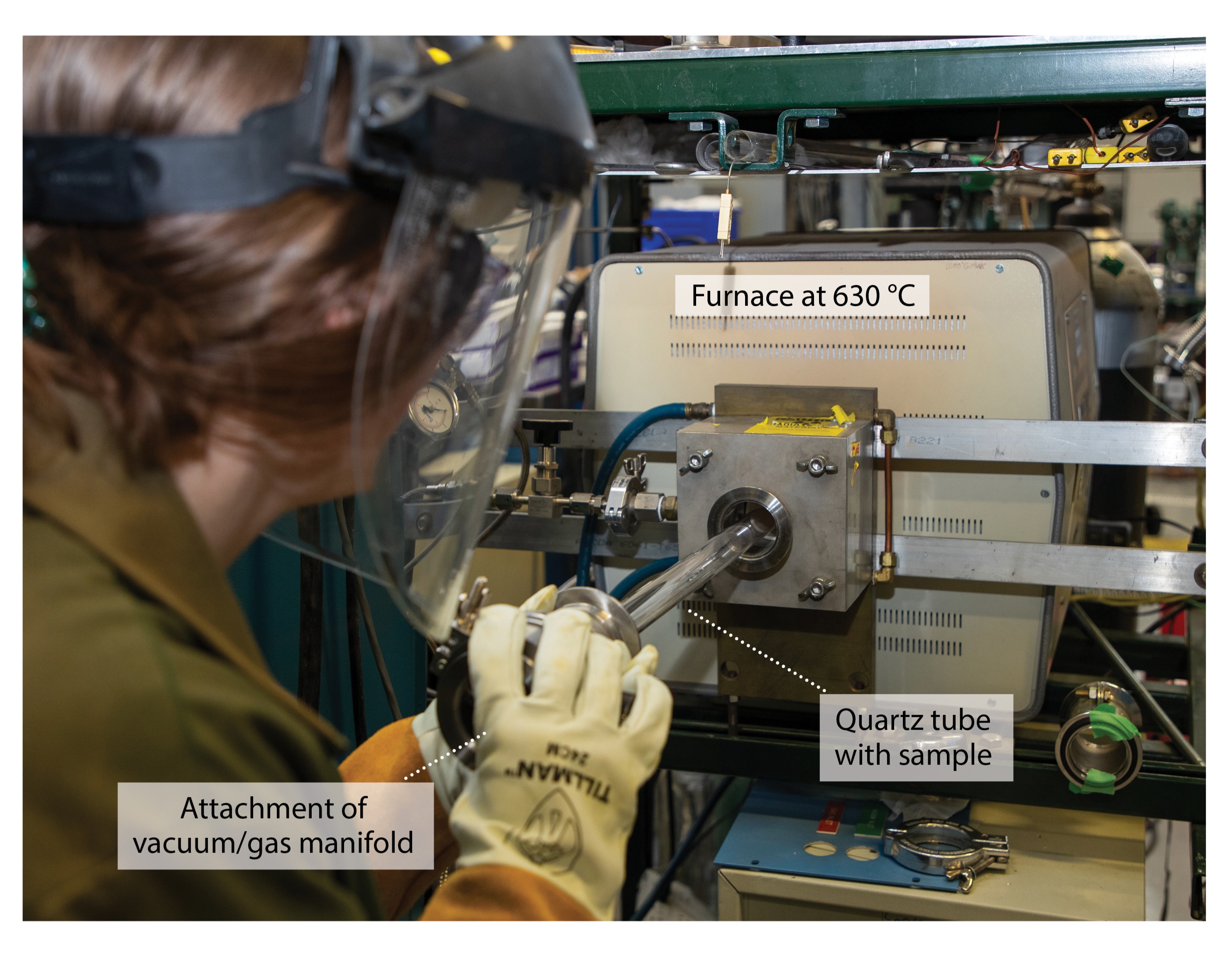
Hydrogen charging and in situ elastic lattice strain measurements
Our hypothesis is that hydrogen lowers the energy necessary for the initiation of line defect movement, thus causing early onset plasticity. To test this, in situ neutron diffraction data were obtained during compression tests using through-thickness textured clock-rolled depleted uranium. Although hydrogen embrittlement tests are typically conducted using tension and discussed in terms of reductions in ductility, the initiation of defect movement is a phenomenon that occurs regardless of the direction of externally applied stress. Because compression specimens are simple cylinders compared to the more complex geometries required for tension specimens, compression was chosen for this initial study. To circumvent the low solubility of hydrogen in uranium at room temperature, hydrogen was introduced during heat treatment at an elevated temperature and “quenched” into place using a well-established hydrogen-charging process. To achieve this process, samples were placed in a quartz tube sealed at one end and attached to a vacuum-gas manifold at the other end (Fig. 4). All samples were first exposed to vacuum at 630 °C for two hours to remove any hydrogen from manufacturing processes. Hydrogen-charged samples were then exposed to a known volume and pressure of a gas mixture containing 99% argon and 1% hydrogen at 630 °C for one hour, while uncharged samples remained in vacuum under identical conditions (without hydrogen). Both the hydrogen-charged and uncharged experiments were concluded by breaking the quartz tube in water. This “quenching” step reduces the energy and reaction time available for uranium hydride formation.
Neutron analysis at LANSCE
Once heat treatment with and without hydrogen was performed, samples were sent to the Los Alamos Neutron Science Center (LANSCE). The texture of the specimens was first measured using the High-Pressure-Preferred Orientation (HIPPO) diffractometer, then sent to the Spectrometer for Materials Research at Temperature and Stress (see SMARTS at LANSCE). Using SMARTS, the samples were compressed, and neutron diffraction data was collected in situ. For these experiments, if defect movement initiated on a particular set of planes, a transition to plastic strain would be observed. It was hypothesized that this transition to plastic strain, indicative of the initial movement of defects, would occur at lower stresses in hydrogen-charged specimens.
True stress-strain curves from the in situ experiments are shown in Fig. 5. The hydrogen-charged sample was only exposed to 3% strain, rather than the 5% strain the uncharged sample was subjected to, due to time constraints on the beamline. Although the hydrogen-charged sample exhibited slightly higher stresses for a given strain than the uncharged samples, the differences are not large enough to be considered significant.

Effect of hydrogen on stress relaxation of alpha-uranium
The initial theory guiding this work was that hydrogen would cause early onset plasticity, or divergence from elastic behavior, due to a reduction in lattice friction it induced. Therefore, in addition to the macroscopic stress-strain behavior in Fig. 5, elastic lattice-strain data was collected at every “serration” visible in the curve (indicated by a gray dashed box in Fig. 5). During the in situ experiments, the crosshead of the mechanical testing frame was paused for around 15 minutes, when neutron diffraction measurements were obtained. During this pause, the sample was held at a constant displacement, but exhibits a relaxation, or reduction, in stress. Once a diffraction dataset was obtained, the sample was then loaded again until the next pause—this stress relaxation followed by re-loading appears as serrations in the stress-strain data in Fig. 5. Unfortunately, the lack of significant difference between the elastic lattice-strain evolutions of the hydrogen-charged and uncharged samples means our hypothesis could not be proved by this experiment.
Typically, stress data obtained during diffraction holds is ignored as it is not the primary focus of the in situ experiments. However, valuable information about the motion of defects can be gleaned during stress relaxation. Rather than graphing stress as a function of strain, the same stress data shown in the gray box in Fig. 5 can be graphed as a function of time during the pause for diffraction collection, showing that stress decreases exponentially until reaching an asymptote (Fig. 6).
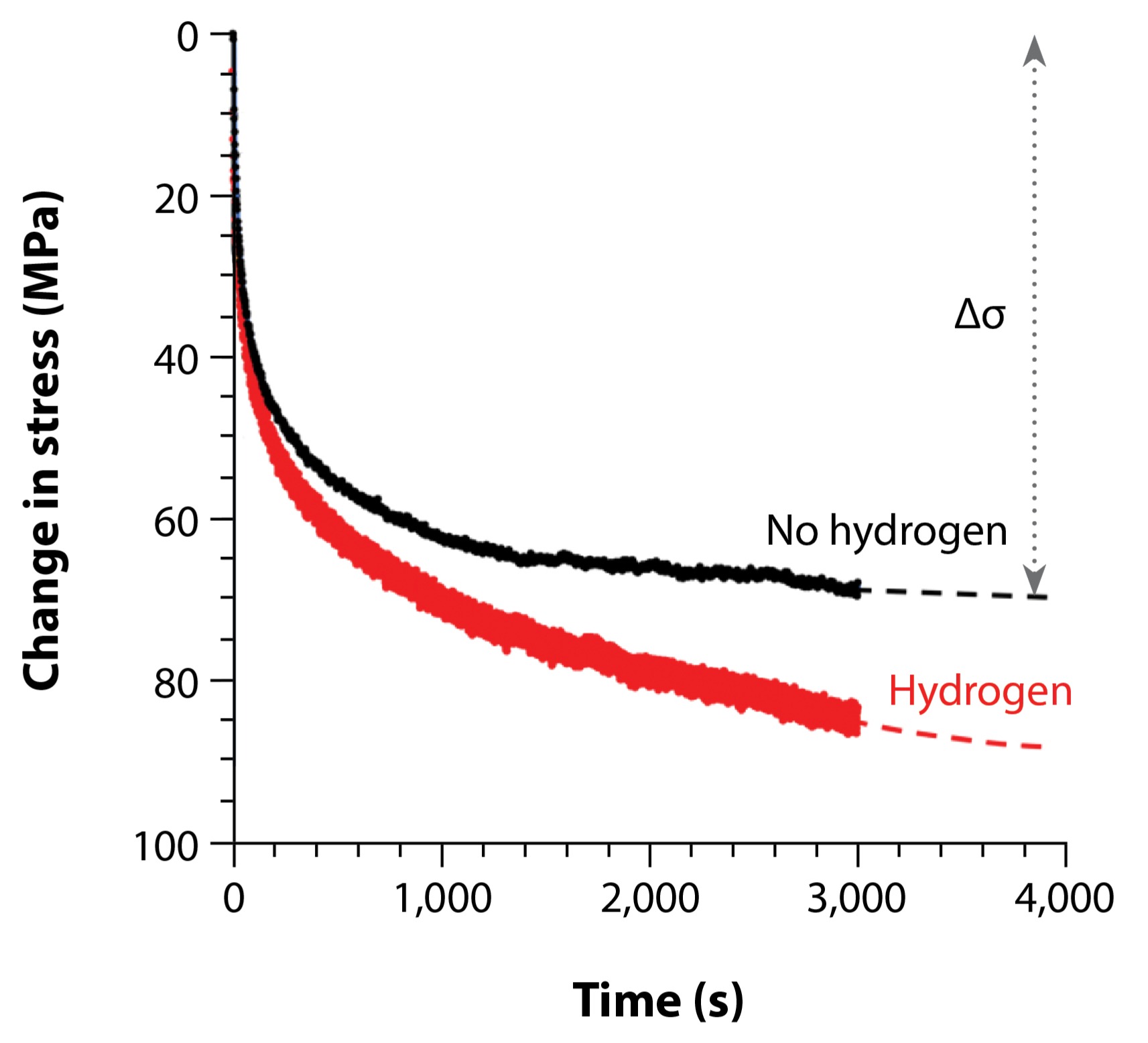
Preliminary analysis of the stress-relaxation behavior during each of the “serrations” indicates that hydrogen-charged samples exhibit a larger change in stress during the hold than uncharged samples. A greater change in stress reflects a greater mobility of defects and, therefore, shows that hydrogen charging has resulted in a greater mobility of defects in this manufacturing condition of alpha-uranium. This enhanced stress relaxation due to hydrogen has been observed in more common engineering materials with high-symmetry crystal structures such as steels. However, to the author’s knowledge, this is the first observation of hydrogen impacting stress relaxation, and therefore defect mobility, in alpha-uranium. This enhanced mobility of defects due to hydrogen could be due to a change in the structure of defects or an effect on the barriers to defect motion. Future work could investigate these mechanisms by coupling advanced in situ microscopy techniques with molecular dynamics models of defects with and without hydrogen. Other work could also examine the effect of longer hydrogen exposures on strain-rate sensitivity.
Summary
Uranium affords a unique opportunity to study the effect of hydrogen on the deformation of metals due to the reduced number of deformation mechanisms available in its low-symmetry structure in the alpha phase. This work set out to understand the effect of hydrogen on the onset of defect movement, also known as the transition from elastic to plastic behavior. This hypothesis was initially tested by measuring in situ lattice strains on families of planes during compression at the SMARTS neutron beamline at LANSCE. Unfortunately, this in situ elastic lattice-strain data did not provide any direct evidence that hydrogen affects the initiation of defect movement on individual lattice planes. However, by using stress-relaxation data that is typically ignored, it was observed that hydrogen significantly increased stress relaxation during pauses in displacement. This enhanced relaxation indicates increased defect mobility due to hydrogen. To the author’s knowledge, this is the first observation of the impact of hydrogen on the stress-relaxation behavior of alpha-uranium. Understanding the effect of hydrogen on deformation in a low-symmetry crystal structure will not only provide crucial insights into the effect of hydrogen on deformation mechanisms in uranium but will also provide a fresh perspective on the influence of hydrogen on metallic systems more broadly.

Mary O'Brien
Mary O’Brien was a Seaborg Institute Postdoctoral Fellow from April 2021 to April 2023 under the mentorship of Samantha Lawrence. She is currently a staff scientist on the Materials Compatibility team in the Sigma Manufacturing Sciences division at Los Alamos National Laboratory. Her research focus is understanding hydrogen embrittlement and corrosion phenomena in a wide variety of metallic systems using advanced characterization and mechanical testing methods, with a current focus on actinide materials.
Acknowledgments
This work is a collaborative effort with several other researchers: Samantha Lawrence, Jason Cooley, Allie Glover, Bjørn Clausen, Donald Brown, Robin Pacheco, Dan Savage, and Sven Vogel all contributed to and played vital roles in this ongoing project. Financial support was provided by the U.S. Department of Energy through the LANL/LDRD program and the Seaborg Institute for the postdoctoral fellowship to Dr. O’Brien.
Further reading:
- K.O. Findley, S.K. Lawrence, M.K. O’Brien, “Engineering challenges associated with hydrogen embrittlement in steels,” in Encyclopedia of Materials: Metals and Alloys, Elsevier, 2022, pp. 235–249.
- P.J. Ferreira, I.M. Robertson, H.K. Birnbaum, “Hydrogen effects on the interaction between dislocations,” Acta Mater., 1998, 46, 5, 1749.
- H.K. Birnbaum, P. Sofronis, “Hydrogen-enhanced localized plasticity—a mechanism for hydrogen-related fracture,” Mater. Sci. Eng. A, 1994, 176, 1–2, 191.
- M.L. Martin, M. Dadfarnia, A. Nagao, S. Wang, P. Sofronis, “Enumeration of the hydrogen-enhanced localized plasticity mechanism for hydrogen embrittlement in structural materials,” Acta Mater., 2019, 165, 734.
- C.S. Barrett, M.H. Mueller, R.L. Hitterman, “Crystal structure variations in alpha uranium at low temperatures,” Phys. Rev., 1963, 129, 2, 625.
- J.S. Morrell, M.J. Jackson, Eds., Uranium Processing and Properties. New York, NY: Springer New York, 2013.
- R.J. McCabe, L. Capolungo, P.E. Marshall, C.M. Cady, C.N. Tomé, “Deformation of wrought uranium: Experiments and modeling,” Acta Mater., 2010, 58, 16, 5447.
- D.W. Brown et al., “Temperature and direction dependence of internal strain and texture evolution during deformation of uranium,” Mater. Sci. Eng. A, 2009, 512, 1–2, 67.
- C.A. Calhoun, E. Garlea, T. Sisneros, S.R. Agnew, “Effects of hydrogen on the mechanical response of α-uranium,” J. Nucl. Mater., 2015, 465, 737.