Advancing Understanding of Actinides in Aqueous Media
- Zach Jones
Advancing our understanding of actinides in aqueous media could help solve many outstanding problems in actinide science. Almost all aspects of technologically relevant actinide chemistry rely—at some point—on aqueous actinide processing. The impact spans from large-scale plutonium metal production to actinide environmental monitoring and efforts to achieve energy security using nuclear power. Unfortunately, we have a real knowledge gap in this area—in particular, speciation and reactivity of actinides in industrially relevant aqueous solutions is often poorly defined. One example is actinide species dissolved in buffered ammonium acetate-acetic acid solutions—beyond a few focused studies, this commonly used stock solution is poorly characterized because of the inherent challenges associated with handling highly radioactive actinides. Interpreting spectroscopic results from actinides in this complicated aqueous environment is also challenging compared to well-defined organic solutions and solid-state crystals. Nevertheless, the absence of knowledge in this area is surprising given that actinide-containing ammonium acetate-acetic acid solutions facilitate small-scale production of actinide metals, serve as starting materials for labeling chelators with alpha-emitting radionuclides for therapeutic applications, and are used in both aqueous synthesis and, to a more limited extent, actinide separations.
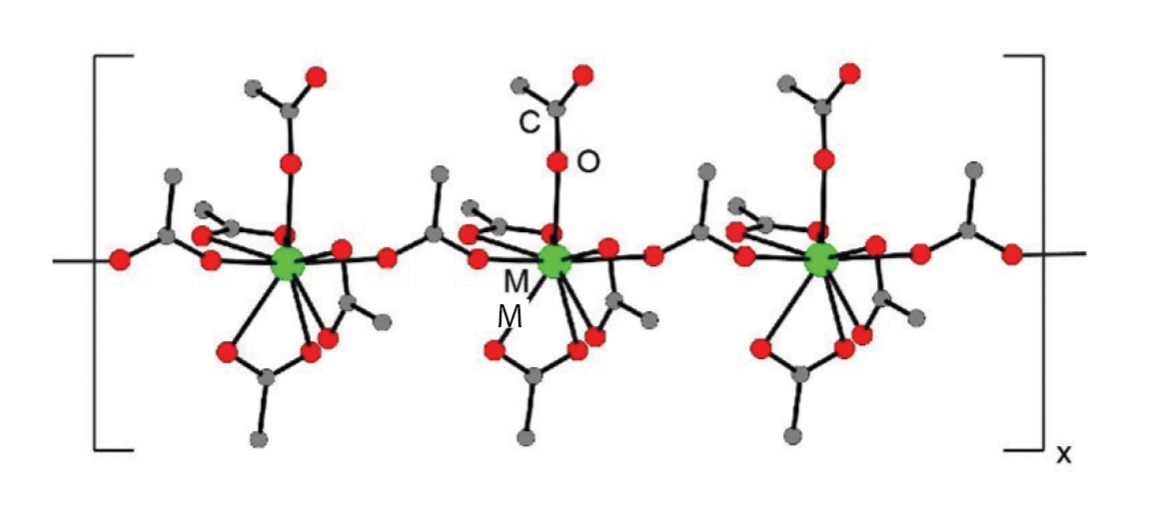
Crystal structures
X-ray absorption spectroscopy
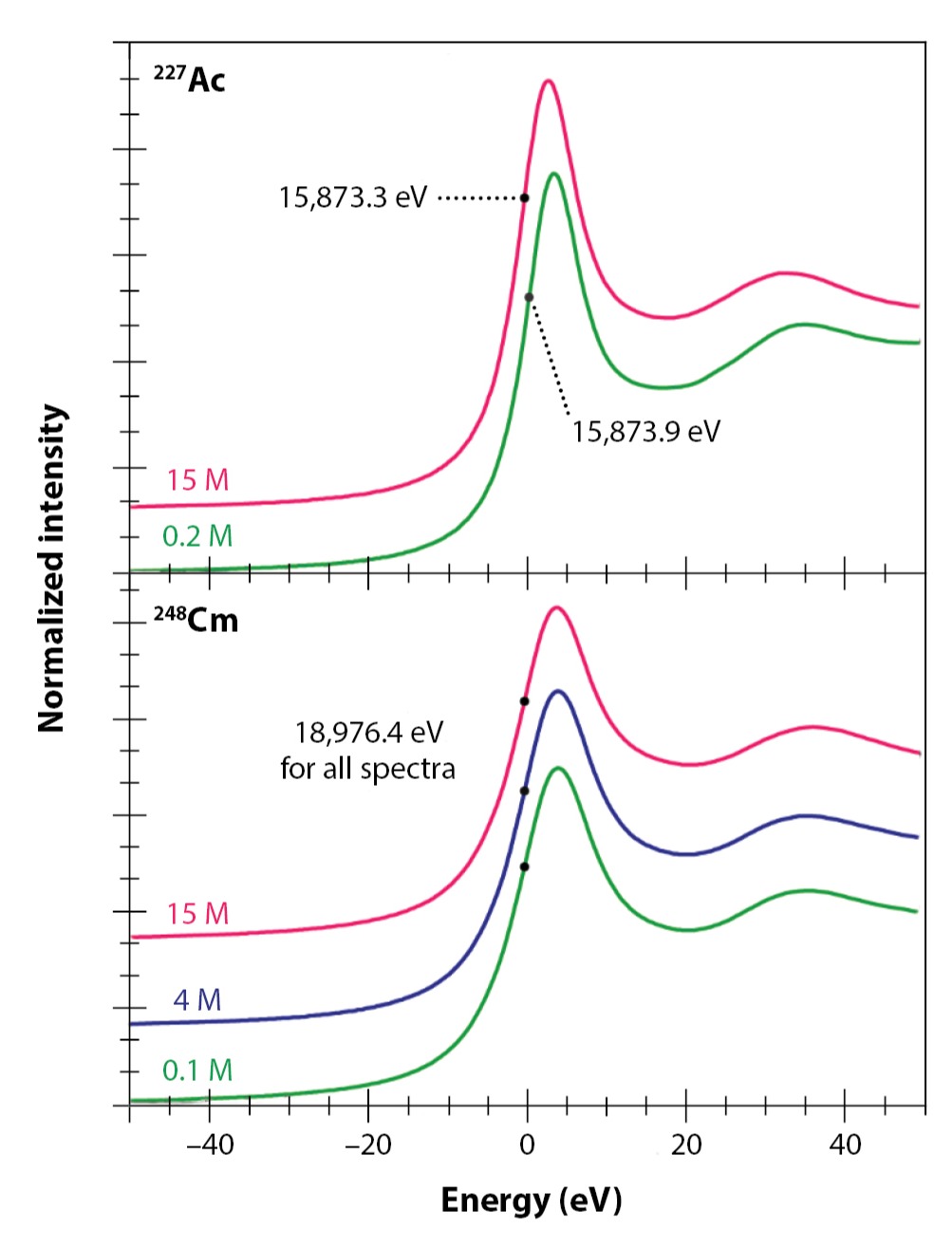
XANES results
XANES alone did not offer substantial insights, as it is only sensitive to the oxidation state and electronic structure of the absorbing atom. Therefore, we turned to EXAFS to help determine the structure around the absorbing atom.
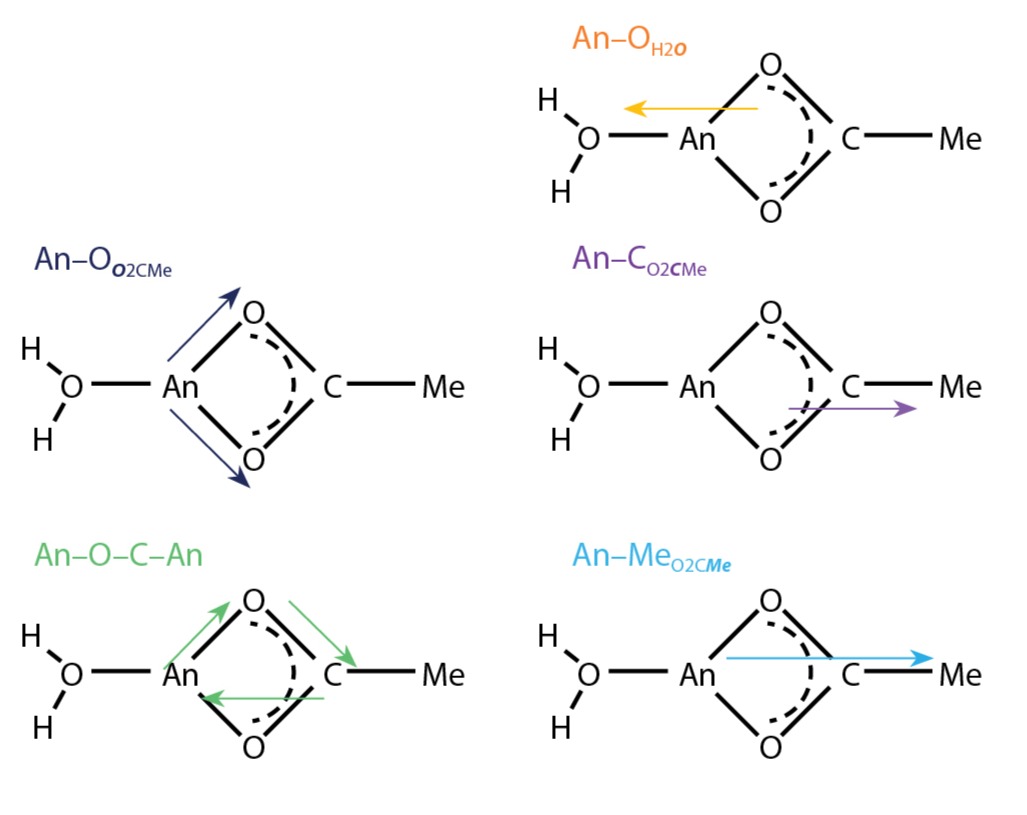
EXAFS results
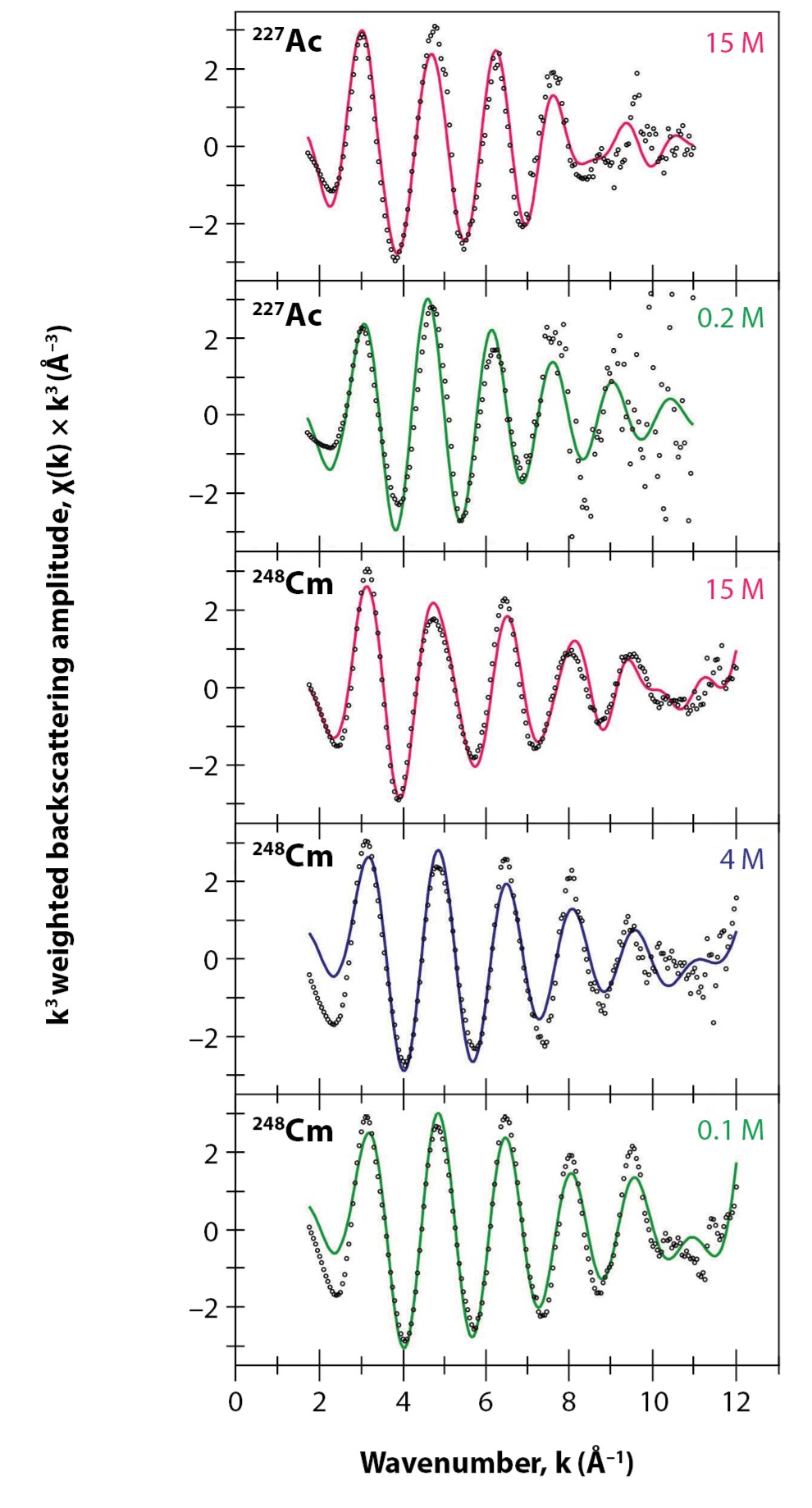
Small changes in Lewis acidity impact actinide coordination chemistry
Our acetate studies align with the common assumption (and a limited number of experimental observations) that actinide(III) complexation is directly influenced by Lewis acidity (i.e., the strength of their attraction to electron donors). Curium, a stronger Lewis acid, showed an inner coordination sphere populated by approximately four acetate ligands in concentrated solutions where acetate ions were in high abundance. For the larger actinium cation, a weaker Lewis acid, the structure contained only three acetate ligands, formulated as neutral Ac(H2O)6(acetate)3.
Summary
A range of X-ray absorption spectra were collected for actinium(III) and curium(III) in aqueous, buffered solutions of ammonium acetate-acetic acid at three different concentrations relevant to current laboratory applications. At low concentrations, the structures were found as the known aquo ions, Ac(H2O)93+ and Cm(H2O)83+. At high concentrations, where acetate ions are abundant, the actinium species was characterized as neutral Ac(H2O)6(acetate)3, whereas the curium solution showed an inner coordination sphere populated by approximately four acetate ligands. This increased affinity is likely due to the higher Lewis acidity of the curium(III) ion, which has a smaller ionic radius.
Better characterization of these aspects of aqueous actinide coordination chemistry would provide researchers with critical information about how active species in solution change with conditions, potentially having significant impact. We hope that this work motivates further study of actinides in relevant aqueous solutions.
Zach Jones
Originally from Portland, Oregon, Zach received a BS in chemistry while conducting undergraduate research on inorganic solid-state superconducting materials with Prof. David Johnson at the University of Oregon. Zach then attended the University of California, Santa Barbara for his PhD work, probing structure-property relationships of nanoscale heterogeneous catalysts with Prof. Susannah Scott. During one of his many synchrotron trips at the Stanford Synchrotron Radiation Lightsource, Zach met Stosh Kozimor, which led to the postdoctoral position and research described herein. Zach is now a Scientist in Sigma division with Fabrication Manufacturing Science (Sigma-1).
Acknowledgments
Many thanks to Maryline G. Ferrier, Benjamin W. Stein, Laura M. Lilley, Stosh A. Kozimor, Maksim Y. Livshits, David H. Woen, Elodie Dalodière, Veronika Mocko, Frankie D. White, Karah E. Knope, Brian L. Scott, and Jennifer N. Wacker for their contributions. These contributions included project conceptualization, writing, editing, acquisition of XAS data, single crystal structure refinement, actinide purification, and optical measurements. We also thank the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Heavy Element Chemistry program (2020LANLE372) and LANL's LDRD-DR (20180005DR and 20190364ER). Postdoctoral support was provided by the Glenn T. Seaborg Institute. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, was supported by the U.S. DOE, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515.
Further reading:
- Z.R. Jones, M.Y. Livshits, F.D. White, E. Dalodiere, M.G. Ferrier, L. M. Lilley, K.E. Knope, S.A. Kozimor, V. Mocko, B.L. Scott, B.W. Stein, J.N. Wacker, F.D. White, “Advancing understanding of actinide (III) (Ac, Am, Cm) coordination chemistry in ammonium acetate and acetic acid buffered aqueous stock solutions,” Chem. Sci., 2021, 12, 5638.
- M.G. Ferrier, B.W. Stein, S.E. Bone, S.K. Cary, A.S. Ditter, S.A. Kozimor, J.S. Lezama Pacheco, V. Mocko, G.T. Seidler, “The coordination chemistry of CmIII, AmIII, and AcIII in nitrate solutions: an actinide L3-edge EXAFS study,” Chem. Sci., 2018, 9, 7078.
- M.G. Ferrier, B.W. Stein, E.R. Batista, J.M. Berg, E.R. Birnbaum, J.W. Engle, K.D. John, S.A. Kozimor, J.S. Lezama Pacheco, L.N. Redman, “Synthesis and characterization of the actinium aquo ion,” ACS Cent. Sci., 2017, 3, 176.