Determination of 99Tc
in Urine using EIChroM TEVA® discs
Buzz Vickery and Tabitha A. Liebrecht
Severn Trent Laboratories
Richland
2800 George Washington Way
Richland, WA 99352
As a commercial laboratory STL Richland has the need for methods that are both cost- and time-efficient. This presentation will focus on a separation scheme for 99Tc in urine using EIChroM TEVA® discs and present the improvements in tracer yield, turnaround time, waste minimization and cost savings as compared to ion exchange columns.
A
sample size of 300 mL of urine is poured into a flask with the appropriate
tracers added. The sample is then
subjected to evaporation, wet acid digestion and muffling. The sample is treated to ensure oxidation of
Tc to the +7 oxidation state. Each
sample is then passed through an EiChrom TEVA® disc. The TEVA® disc is rinsed with 0.01M HNO3 to
remove all possible alpha-emitting interferences. The TEVA® disc is rolled into a LSC vial, mixed with 15 mL of Beckman
Ready-Gel and counted for 60 minutes by liquid scintillation counting.
Tracer
yields have improved ~10% (ave=96.1%, n=63, σ=0.066),
with a ~15% improvement in MDA. The
range for tracer yields are 70% - 104% with 85.7% of the samples being above
90%. There is also 60% less acidic waste
being generated with the TEVA® disc method as compared to the ion exchange method. The analysis time decreased from 48 hours to
12 hours after switching to the TEVA® disc method.
Different
sample preparation variables were experimented with in this study, which
occurred between 11/30/99 – 1/13/00.
Some of the samples were repeatedly wet-ashed with 30% H2O2
and concentrated HNO3 (batches 9340413, 9340415, 9344288,
0007198). Other samples were wet-ashed
and heated between 200°C and 500°C (batches
9348468, 9348470 and 0007201). Some
samples were less than 2 weeks old (batches 9340413, 9340415, 9344288, 9347455
and 9347454) while others were 12-months old (batches 0007201 and 0007198). Samples were either spiked at the beginning
of sample preparation (batches 9340413, 9340415 and 9344288) or at the
beginning of chemical separation (batches 9344290, 9347455 and 9347454), there
was very little difference between recoveries of these samples. Muffled samples had excellent recoveries
(ave.=94.5%, n=16, σ=5.19) at all temperatures tested. Non-muffled urine had an average percent recovery
of 95.7%, tSIE of 316, and a Luminescence (Lm) value of 6.8 (Table 1), while
muffled samples had average values of 94.8%, 407.1 and 1.7, respectively (Table
3). The most noticable advantage of
muffling samples (old or new) is that they have higher tSIE and lower Lm
values, which translates into higher efficiency and lower interference. Another test was to see if one (1) liter of urine
would overwhelm and cause breakthrough of 99Tc on the TEVA® disc (Table 2). There were a total of 52 samples tested, 16 of these samples were
matrix blanks. These 52 data points are
shown graphically in Figure 1. There
were also 22 instrument blanks, which were used to calculate the MDA (Figure
2). The distribution coefficient for
the TEVA® disc
was developed by EIchroM Industries.
Table
1: Table of Tracer Recovery (artificial, fresh, and 12 month old urine)
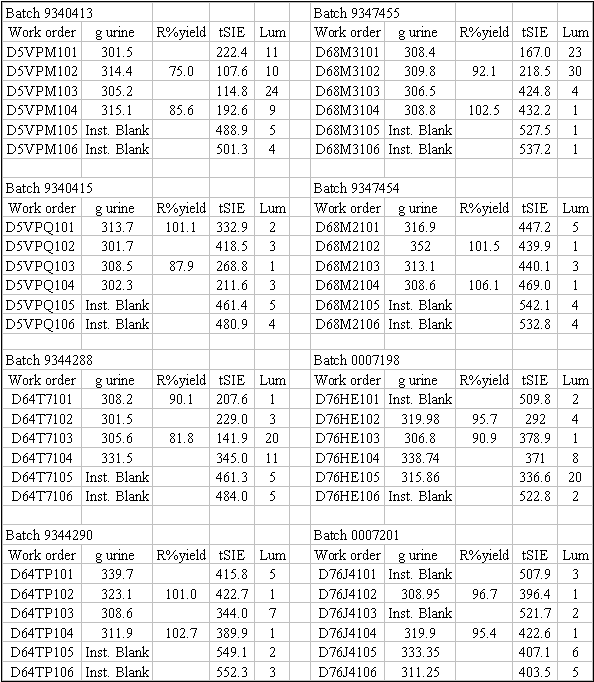
R%yield = Radiochemical
Yield = Tc99 recovered / Tc99 added * 100
Figure
2: Table of Tracer Recovery for
High-Volume Samples
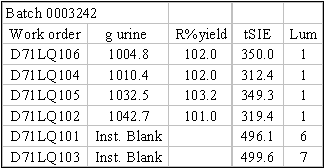
Figure3: Table of Tracer Recovery for
High-Temperature Samples
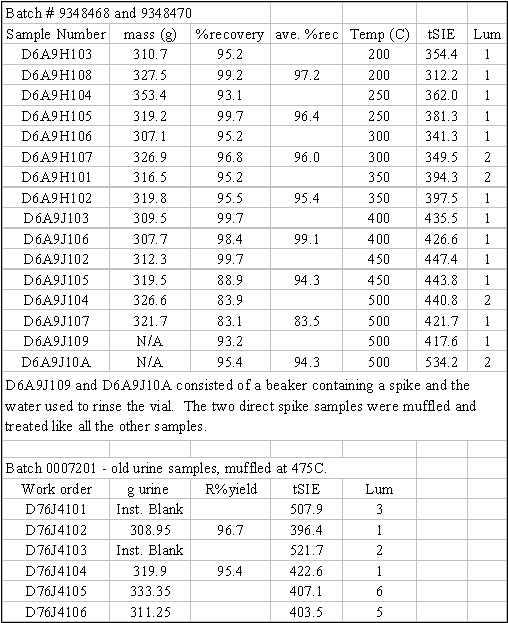
Figure 1: Plot of all urine data points

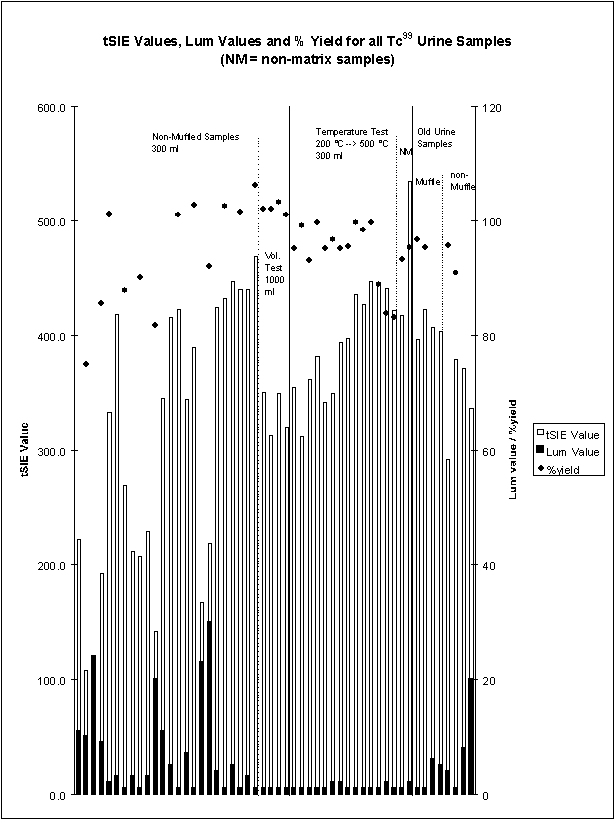
Figure 2: MDA caluculation
Based on initial calculations the MDA is 4.69
pCi/L for a 300ml urine sample, and 1.47 pCi/L for a 1000ml urine sample. The mean and the standard deviation of the
blanks used to calculate the MDA is:
RB = 22.86, 0.717
E = 0.8871, 0.0233
VA = 0.3101, 0.0104
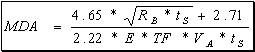
Where MDA =
Minimum Detectable Activity of the sample
RB =
Count rate of detector background (in cpm)
E =
Detector efficiency
ts =
Count time for analysis
TF = Transmission factor = 1
VA
= Sample aliquot volume