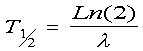Biological Half life of Iodine in Normal and Athyroidic persons.
Gary H. Kramer†, Barry M.
Hauck† and Michael C Chamberlain‡
†Human Monitoring Laboratory, Radiation
Protection Bureau, 775 Brookfield Road, Ottawa, Ontario K1A 1C1, Canada
(Gary_H_Kramer@hc-sc.gc.ca, www.hc-sc.gc.ca/ncrc/)
‡Division of Nuclear Medicine,
Ottawa Civic Hospital, 1053 Carling Avenue, Ottawa, Ontario K1Y 4E9, Canada.
INTRODUCTION
The biological half-life of iodine
in the human thyroid might be expected to be, on average, an invarying
quantity. In 1959 the International
Commission on Radiological Protection (ICRP) recommended that the biological
half-life of iodine should be138 days (ICRP 1960). This was revised downwards in 1978 to a value of 120 days (ICRP
1979) and yet again in 1989 to a value of 80 days (ICRP 1989). Similarly, the amount of iodine retained by the
thyroid has not changed from 0.3 over this period.
The
metabolism of iodine by an adult according to the ICRP (ICRP 1989) is that 0.3
of the initial intake is taken up by the thyroid and 20% goes to faecal
excretion, the biological half times are: blood, 0.25 d; thyroid, 80 d; rest of
the body, 12 d. The recycling of iodine
can be best be described by a two compartment model but this is not seen when
using 131I as the short radiological half-life precludes the
resolution of the two compartments.
According
to this model, person without a thyroid gland would be expected to excrete all
iodine immediately, or at least within a very short period of time as there is
some iodine retention by the salivary glands.
There are two main types of salivary glands - the major salivary glands
and minor salivary glands. The three types of major salivary glands are the
parotid glands, submandibular glands, and sublingual glands. There are two of
each type - one on the left side and the other on the right. The parotid glands
are the largest salivary glands and are found on each side of the face, just in
front of the ears. They overlie the jaw joint and would not contribute many
photons to a detector placed in front of the thyroid gland.
The
submandibular glands are the next largest salivary glands and are found on
either side of the neck, under the chin and tongue area. The sublingual glands
are found deeper in the neck than the submandibular glands, under either side
of the tongue. There are about 600-1,000 minor salivary glands, which are too
small to see without a microscope. These minor salivary glands are located
beneath the lining of the lips, tongue, hard and soft palate, inside the
cheeks, nose, sinuses, and voicebox.
These glands are closer to a detector placed in front of the thyroid
gland, especially if it has a large diameter, and could contribute
photons. However, as the biological
half life of the salivary gland is approximately 10 hours (Nishizawa et al.
1985) one would expect the athyroidic subjects’ retention to be governed by
this pathway
In 1996 the Human Monitoring Laboratory (HML)
and the Ottawa Civic Hospital collaborated to measure the biological retention
of iodine in normal and athyroidic patient by sequential measurements of the
thyroid or whole body retention of 131I. The advantages of the two organisations cooperating to perform
this study was that no person received an unnecessary exposure to radioactive
materials as all the participants in this study would have received the
diagnosis or therapeutic doses of 131I regardless of their
participation. The disadvantages were
that the age/gender mix of the subject pool could not be pre-determined.
The
study commenced in March 97 and finished in December 1999. The results have been compared to the ICRP
recommendations and the ICRP metabolic models.
METHODS AND MATERIALS
Volunteers: The subjects participating
in this study were solicited at the Ottawa Civic Hospital by the nursing staff
following either a diagnostic or therapeutic administration of 131I. Each volunteer came to the HML for up to six
counts spanning up to six weeks.
Volunteers were compensated for travelling expenses for each visit to
the HML and each completed a consent form prior to the first count.
Low Background Counting
Chamber: The low background counting chamber which
houses the whole body/thyroid counter was constructed in 1959 by the Dominion Bridge Company using material
supplied by the Steel Company of Canada. Prior to construction, samples of
steel were sent to the University of Toronto, Physics Department, to test for
radioactive contamination. Evidence of some contamination (mostly 137Cs
and 60Co) was found that was attributed to radioactive fallout from
atomic bomb testing in the 1940's and 50's. The chamber was installed in the
Radiation Protection Bureau in 1960 and have been used in the Human Monitoring
Program of the Health Canada for 40 years.
The
thickness of each chamber wall, floor, and ceiling is 0.2 m, and the
approximate weight of the chamber is 100 tons. The wall thickness is sufficient
to reduce the gamma rays from naturally occurring radioactivity in the
surrounding building materials by a factor of about 1000, and the cosmic rays
to about 60% of their unshielded intensity. The inner surfaces of the rooms are
covered by 6.3 mm of lead which reduces the background, below 0.1 MeV, by a
factor of two.
The inside dimensions of the chamber is 1.52m by 2.13m by
2.13m. The chamber is equipped with double doors operated by electric motors
controlled from the laboratory. There is a second control in the chamber which
can be used to open the doors from inside in case of emergency. An intercom is
also provided for communication between subject and operator, as well as music
to relieve the tedium of lengthy counting periods. Subjects may also be viewed
through a large water filled window of dimensions 0.3m by 0.46m by 0.6m wide.
Detector systems: The whole body counter is equipped with six
NaI(Tl) detectors combined in two triangular arrays. The upper array consists of three detectors scan above and the
lower array consists of three detectors scan below the subject. The upper array is on a moveable arm that
can be raised from the bed surface to the roof of the counting chamber. The lower array is in a fixed geometry 12 cm
below the bed.
Each
detector array is powered by an independent high voltage supply. The signal from each detector is processed
by a preamplifier. The three for the
upper array are connected to a dual sum invert amplifier and the other three
for the lower array to another. These
modules sum the incoming signals into single signals. The signals are then processed by two separate amplifiers that
are connected to a multichannel buffer.
Spectral analysis is performed on a computer using using
EG&G’s GDR software custom modified for the HML
Each
detector in the upper and lower arrays is a cylindrical NaI(Tl) crystal that is
nominally 12.7 cm in diameter and 10.2 cm high. The crystal is optically coupled to a low background
photomuliplier tube. The outer casing
of the detector is stainless steel 304 (Fe, 70%; Cr, 19%; Ni,11%; specific
gravity, 8.02) which is 0.635 mm thick.
The transmission of photons through the outer
casing at 100 keV 83% rising to 97% at 1000 keV.
Only
one detector of the upper array is used for thyroid counting, the other two
being removed from the array by disconnecting the signal cables. Normally the detector is placed centred over
the supine subject’s thyroid gland at a distance of 14 cm. However, some of the subjects had such high
activities that requiring the detector had to be raised to reduce the dead time
to manageable levels.
The
detector arrays scan the subject for normal whole body counting, but the
scanning detector geometry can also identify the location of a radionuclide
that is not homogeneously distributed in a person (or phantom) using the Multi
Channel Scaling (MCS) mode of the Whole Body Counter. The latter mode was used
in conjunction with the thyroid count mode.
Counting Efficiency: The counting
efficiency for 131I thyroid counting was determined using the BRMD
thyroid phantom (Kramer et al 1996a, 1996b) placed as the neck section of a
Reference Man BOMAB phantom (Kramer et al 1991) using 133Ba as a
surrogate for 131I. The
thyroid counter was also calibrated using a BOMAB phantom containing 133Ba
distributed homogeneously throughout the phantom. The efficiency was determined at the normal counting distance (14
cm) and at larger distances.
Counting Protocol: All subjects were
measured in the whole body counter in a supine position. The first count was a
whole body count with the detectors scanning over the subject. The counts were acquired in multi channel
scaling mode simultaneously with the normal acquisition. The second count was a thyroid count where
the subject remained supine but also extended the neck to raise the thyroid
gland above the collar bones.
The
counting regime for diagnostic patients was begun within a week of the initial
administration of 131I with repeat counts being performed at
approximately 7 day intervals; however, the regime for therapeutic patients had
to commence as quickly as possible to measure the rapidly excreted iodine. Repeat counts were daily (where possible)
for the first three to four and the last counts were at a weekly interval. The detector array was raised for these
patients.
Half-Life Determination: The effective
half life is given by:
|
|
|
1 |
where 8eff is the effective decay rate (d-1), 8rad is the radioactive decay rate (d-1),
and 8biol is the biological decay rate (d-1). 8eff is obtained from the linear regression of
Ln(thyroid activity) as a function of time,
8rad is
8.03 days (reference) and so 8biol can
be obtained from Eqn. 1. The biological
half-life is then obtained from:
|
|
|
2 |
where T½is the half-life
(d) and 8 is the decay rate (d-1)
RESULTS AND DISCUSSION
Volunteers: Diagnostic patients were
given about 400 kBq. The therapeutic
patients are in two groups: the higher amount of 131I is for
treatment (150 GBq), the lower amount (150 Mbq) of 131I is for
confirmation treatment was successful.
Biological Half-Life: The 131I
retention as a function of time can be described by a single compartment and
this was observed by all the normal subjects measured at the HML. By contrast, the athyroidic patients showed
a different pattern of retention. This
is surprising on two counts, first that there was any retention at all, and
secondly because this retention is described by a two compartment model.
The
data shows that the biological half-lives of the normal patients varied from
11.4 to over 4,000 days. Similarly the
uptake values vary from 3% to 63%.
Despite the fact that all these subjects received 131I as a
part of a medical diagnosis subsequent evaluation of their condition showed
them all to be normal. Therefore, they
are considered to representative of a normal healthy North American population.
The
athyroidic patients fall into two distinct uptake groups. These correspond to the subjects who
received sufficient 131I to ablate the thyroid gland and the other
group, with the slightly higher uptake values, those subjects who received a
confirmatory amount of 131I that the ablation (previously done) was
successful. The short term retention is
probably due to salivary gland retention, but the mechanism of the longer term
compartment is unknown.
Table
1 summarises all the data. Subject
MBH who has an unusually long
biological half-life for iodine has been eliminated from the data set as an
outlier and is not considered in the subsequent statistical analysis.
Table
1shows that the average biological half life is 57.3 " 5.4 days. Direct comparison with the ICRP value of 80 days is difficult as
there are no uncertainties associated with this value. A comparison can be made if it is assumed
that the uncertainty on the ICRP value is similar to the one obtained in this
study. Performing a t-test, to test the
null hypothesis that there is no difference between the two values, one obtains
a t-value of 10.86 (t-crit = 2.33, P = 0.000) strongly suggesting that the null
hypothesis be rejected. It is likely
that the decrease in biological half-life of the North American group that
participated in this study is a continuation of the trend identified above and
is likely due to changes in food and its additives. Iodine is now plentiful in the diet and the body has no reason
the retain the material for long periods of time. Similarly the average fractional uptake of the thyroid seems to
have declined from the ICRP recommended value of 0.3 to 0.22 " 0.02.
Retention model: Based on the findings
of this study the retention model of iodine can be written as:
|
|
|
3 |
CONCLUSIONS
This
study has shown that the biological half life of iodine seems to have decreased
since the ICRP recommended 80 days.
Changing the half life to the value of 57 days and the uptake to 0.22
will not have much effect on the dose delivered by 131I as the
dominant removal pathway is the radioactive decay (8.04 day half-life);
however, for 125I and other long lived isotopes it may be a dose
reduction consideration.
REFERENCES
International Commission on
Radiological Protection. Report of ICRP
committee II on permissible dose for internal radiation (1959), with
bibliography for biological, mathematical and physical data. Health Physics 3-4; 1960-61.
International Commission on
Radiological Protection. Limits for
intakes of radionuclides by workers.
Oxford: Pergammon Press; ICRP
Publication No. 30, Part I; 1979.
International Commission on
Radiological Protection. Age dependent
doses to members of the public from intakes of radionuclides: Part 2 ingestion
dose coefficients. Oxford: Pergammon Press; ICRP Publication No. 67,
Part 2; 1993.
Kramer G.H., Noel L. and Burns
L.C. The BRMD BOMAB Family. Health Physics, 61(6): 895-902; 1991.
Kramer, G.H., Olender G., Vlahovich
S., Hauck B.M., Meyerhof D.P.
Comparison of the ANSI, RSD, KKH and BRMD Thyroid-Neck Phantoms for 125I
thyroid monitoring. Health Physics
70(3):425-429; 1996a.
Kramer, G.H., Gamarnik, K.,Noël, L.,
Burns, L.C.,Meyerhof, D. The BRMD
Thyroid-Neck Phantom: Design and
Construction. Health Physics 71(2):
211-214; 1996b.
Nishizawa, K.; Hamada, N.; Sadayuki,
S. In
Vitro monitoring of salivary 125I. Health Phys. 49(2): 290-295; 1985.
Table 1 Statistical summary of the biological half lives for all subjects
|
|
Normal T½ |
Athyroidic short T½ |
Athyroidic long T½ |
Units |
|
Average |
57.27 |
1.03 |
18.47 |
days |
|
F |
33.12 |
0.53 |
3.44 |
days |
|
F(mean) |
5.37 |
0.19 |
1.15 |
days |
|
N |
38 |
8 |
9 |
|
|
median |
50.70 |
0.81 |
19.17 |
days |