Summary of the Mayak Pa Internal Dosimetry Program.
Melinda P. Krahenbuhl, David M. Slaughter, Scott C. Miller,
and Valentin Khokrykov
The scientists at the First Branch of the Biophysics Institute of the Russian Federation (FIB-1) are engaged in plutonium dose assessment for the workers of the Mayak Production Association. The Mayak PA facility was established in 1948 and was the first plutonium facility in the former Soviet Union. Unlike the US which separated reactor operations, chemical separation and pit production between Hanford, Oakridge and Rocky Flats, the Soviet Union established independent facilities each capable of producing plutonium, separating and milling high enriched plutonium for nuclear weapons. In conjunction with the Soviet Unions rapid nuclear development came an emerging health physics program. This program has unique characteristics from those programs developed in the US. The Joint Coordinating Committee for Radiation Effects Research (JCCRER) was established to explore the health effects of radiation doses, at the Mayak PA and surrounding area. Projects include dose reconstruction of the Techa River cohort and two cohorts established from the Mayak workers.
Project 2.4 deals with the quality control and quality assurance of the databases and the doses recorded, for both internal and external dosimetry of the worker cohorts. The 2.4 team is loosely divided into internal and external dosimetry groups. Project 2.4 started with database verification. First the data control was established. The paper records were transferred to a web based database. Random checks for accurate data entry are preformed, but this exercise does not confirm the quality of the data. This aspect is more complex. Paramount to understanding the internal doses are how the recorded doses were derived. This understanding encompasses sample collection, radiochemical analysis and modeling algorithms. This presentation focuses on the modeling algorithm, but will briefly address collection and analysis.
Urine samples were collected and processed at both the industrial site and the clinic. Samples are collected from individuals who perform at risk tasks or based on supervisor discretion. If a urine sample collected and analyzed at the site was found to have a plutonium concentration over 20 nCi for a 48 hour collection were placed on leave and referred to the clinic for follow-up. Urine samples are also collected from workers who are admitted to the hospital adjacent to the clinic. The primary cause of hospitalization does not need to be associated with radiation exposure for a plutonium bioassay to be preformed.
Samples are processed using bismuth phosphate precipitation. The precipitant is than mixed with ZnS and counted using a radiometer. The process and counting techniques are described in Suslova et al 1996 , Filipy et al 1998, Khokryakov et al 1998. Count times and background changed with time. The minimum detected activity dropped from 6.4 to 0.6 mBq. Typically 200 ml aliquats were processed which results in a minimum detectable activity of 32 to 3.0 mBq/L respectively.
The FIB–1 model has a three-chambered lung that is attached to a 10-chambered mathematical construct. This model results in a system of 13 coupled ordinary differential equations. This system of equations was solved for organ content using Eulers method. Integration of the organ content solution results in the dose imparted to an organ by incorporated plutonium. The relationships for dose are:
![]()
![]()
![]()
where
D is the dose to the organ in rem
Qbody is the plutonium content in the body
M is the mass of the organ
t is the duration of the exposure
t is the time post exposure
G1through G4 are substitution variables used to simplify the equations.
The plutonium content in the body is related to the bioassay measurements as follows.
![]()
where Qo is the bodys’
plutonium content
K is a conversion factor 0.0135 Bq/dpm or 0.000365 nCi/dpm,
Um is the average daily activity measured in urine (dpm), and
Ko is the fraction of plutonium excreted per day of the total plutonium content.
![]()
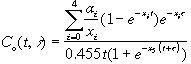
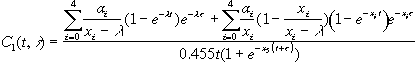
The following criteria is used to determine dose for the individuals in the cohort:
1. If autopsy data exists it is always used.
2. If no autopsy data exist and the individual is deceased, urine bioassays taken within one year prior to death are excluded.
3. The characteristic transportability is selected based on the plant or subplant in which the worker had spent the majority of his of her employment.
4. Pre-1999 the results are reported for only the final sample collection. After 1999 the results from the last three sample collections were averaged for the stochastic effects cohort. This average result is the reported value of the dose after 1999.
Using this algorithm, the doses for 5332 individuals were determined. These doses range from 0 to greater than 1000 Gy, with the majority of the doses falling between 0.0 to 0.1 Gy. The error analysis includes standard counting and Monte Carlo techniques.
Distribution of accumulated equivalent doses due to internalized plutonium
(Gy) n= 5332
|
Organ |
0-0.1 |
0.1-1 |
1-10 |
10-100 |
100-1000 |
> 1000 |
average |
standard deviation |
maximum |
|
Lung |
2173 |
1476 |
1470 |
282 |
34 |
0 |
3.6 |
16.9 |
375.7 |
|
Liver |
2171 |
682 |
1982 |
444 |
53 |
0 |
5.5 |
22.1 |
457.7 |
|
Bone surface |
2170 |
6 |
1183 |
1635 |
309 |
28 |
34.7 |
139.7 |
2888.7 |
Filipy,
R.E.; Khokhryakov, V.F.; Suslova, K.G.; Romanov, S.A.; Stuit, D.B.; Aladova,
E.E.; and Kathren, R.L. Analysis for
actinides in tissue samples from plutonium workers of two countries. J.
Radioanalytical Nucl. Chem. 234:171-174, 1998.
Khokhryakov
V.F.; Kudryavtseva, T.I; Chernikov, V.I.; Suslova K.G.; Orlova, I.A.; Filipy
R.E. A scintillation method for determination of actinide alpha-activity in
samples. J.Radioanal.Nucl. Chemistry. 234:293-295;1998
Suslova,K.G.; Filipy R.E.; Khokhryakov V.F.; Romonov S.A.; Kathren R.L. Comparison of the dosimetry registry of the Mayak Industrial Association and the United States transuranium and uranium registries: a preliminary report. Radiat. Prot. Dosim. 67:13-22;1996.