Optimizing the
NIST Standard Sequential Extraction Protocol Using a Full-Factorial
Experimental Design
Michael
K. Schultz1,
Kenneth G. W. Inn2, and William C. Burnett3.
1. PerkinElmer Instruments, 801 S. Illinois Avenue, Oak Ridge,
TN 37831-0895, michael.schultz@perkinelmer.com, Phone: (843) 841-2446.
2. National Institute of Standards and Technology, Radioactivity
Group, 100 Bureau Drive, Mailstop 8461, Gaithersburg, MD 93001, kenneth.inn@nist.gov,
Phone: (301) 975-5541.
3. Florida State University, Department of Oceanography, Tallahassee,
FL 32306-4230, burnett@ocean.fsu.edu, Phone: (850)
644-6703.
Extended
Abstract
The NIST Standard Sequential Extraction Protocol
We
are developing a Standard Sequential Extraction Protocol, termed the NIST
Standard Sequential Extraction Protocol (NSSEP), for determining the
geochemical fractionation of uranium and plutonium in soils and sediments. Sequential extraction methods are used to
investigate geochemical relationships of actinides and trace metals in soils
and sediments (Tessier, et al. 1979).
These geochemical relationships are used to understand the conditions
under which contaminants may be released to the surrounding environment.
The
sequential extraction approach employs a series of operationally-defined
chemical extractions in an attempt to dissolve selectively discrete geochemical
components of soils and sediments. For
example, analyte metals that may become environmentally available through
changes in ionic strength can be identified by the application of an
ion-exchange solution such as MgCl2. As
a further example, analyte metals that are occluded in or irreversibly adsorbed
to the lattice structure of oxides of Fe and Mn can be identified by the
application of a reducing solution (e.g. hydroxylamine hydrochloride NH2OH·HCl).
Unfortunately, interpretation of sequential extraction results can be complicated by several factors. Although each extraction in the sequence is designed to attack a single geochemical phase, complete specificity is not likely. We developed an experimental plan to evaluate systematically the experimental conditions under which extraction selectivity could be optimized for uranium and plutonium in a NIST Standard Reference Material (SRM). The NSSEP includes five chemical extractant solutions (Table 1) ¾ arranged in a sequence designed to maximize the dissolution of the target phase, while minimizing the destruction of non-targeted phases. Stable metal analyses were combined with radiometric analyses to determine the specificity of reactions (Schultz, et al., 1996, Schultz et al., 1998a, Schultz et al., 1998b, Schultz et al., 1999).
The NSSEP is being developed by NIST to establish a standard method for comparison of the results of sequential extraction experiments. The method is being developed using NIST Standard Reference Materials SRM’s . The experiments described here were conducted using NIST SRM 4357 Ocean Sediment. The Ocean Sediment material is a mixture of contaminated material obtained from the Irish Sea and relatively uncontaminated material collected from the Chesapeake Bay. The resulting standard consists of isotopic plutonium concentrations well in excess of fallout and naturally-occurring isotopic uranium concentrations that are easily measured by alpha-spectrometry.
Table 1 Summary of the extraction sequence
being tested for the development of the NIST Standard Sequential Extraction
Protocol.
|
Desired Fraction |
Extractive Reagent |
Reagent/Sample Ratio (mL/g) |
|
Water
Soluble/ Exchangeables |
H2O/MgCl2 pH 4.5 |
15:1 |
|
Carbonates |
NH4Ac in 25% Hac pH 4 |
15:1 |
|
Oxides
(Fe/Mn) |
NH2OH·HCl in 25% Hac pH 2 (HNO3) |
15:1 |
|
Organic
matter |
30% H2O2 in 0.02 M HNO3 pH 2 |
15:1 |
|
Acid
Soluble |
8M HNO3 |
15:1 |
|
Residue |
Total dissolution |
as needed |
Design of Experiment ¾ The
Full-Factorial Approach
In
this paper, we present some of the fundamentals of so-called Design of
Experiment DEX ¾ as they relate to the
development of the NSSEP. The results of the optimization experiments conducted
are used as examples of the DEX approach.
We
define DEX as a rigorous-systematic approach for solving scientific and
engineering problems so that unambiguous results are obtained at minimum
cost. The main elements of our approach
include:
·
Establishing
criteria for response success.
·
Establishing
a level of statistical significance and eliminating “unimportant” variables.
·
Establishing
high and low settings for variables.
·
Conducting
experiments that simultaneously include three variables.
·
Conducting
auxiliary experiments.
·
Analyzing
the results
Using
the DEX approach, reaction time, reaction temperature, and extractant
concentration were identified as experimental variables ¾ it was determined that changes in these
variables would likely result in identifiable changes in the extraction of
targeted geochemical fractions. The
phase specificity of the extractions was monitored by measuring the
concentration of stable elements Al, Ba, Ca, Fe, K, Mn, Pb, Sr, and Ti in
extractant solutions. Extraction
efficiency was determined at two extreme values for each variable and a
midpoint experiment was included to identify curvature in the relationship. The resulting full-factorial design provides
for an estimation of the effect (change in the % extracted U, Pu or stable
metal), as the independent variables (time, temperature, and reagent
concentration) are varied from a low to a high value. Once the full factorial experiment has been completed, the
response “y” can be estimated mathematically by the equation:
![]() Eq 1
Eq 1
where:
y = response for an individual experimental run.
m = average response over all experimental
runs.
btm = Average effect of
reaction time.
btp = Average effect of
reaction temperature.
bc = Average effect of reagent
concentration.
ctm = Duration of experimental
run.
ctp = Temperature of
experimental run.
cc = Reagent concentration of
experimental run.
 This model includes not only
effects from changes in each variable, but also effects of variable
interactions.
This model includes not only
effects from changes in each variable, but also effects of variable
interactions.
Graphically,
the design becomes a three dimensional figure with the axes representing the
independent variables time, temperature, and concentration (Fig. 1). The origin represents experiments conducted
at the “low” value (coded “-”) for each (e.g., 1 hour, 25°C, 0.1 M MgCl2 for the
exchangeable fraction). The opposite
end of each axis represents the high value (coded “+”) for each variable (e.g.
4 hours, 90°C, and 1 M MgCl2. This paper will illustrate the determination
of the optimum conditions for the sequential extraction of a soil
material. This results in nine total
experimental runs per fraction (including the mid-point). For the NSSEP, the experimental runs were
randomized to minimize the effect of learning curve on the results.
Results
Summary
Using the DEX approach we have completed the optimization of the first NIST material SRM 4357 Ocean Sediment (Table 2).
Our results indicate that over 60% of natural uranium (as U-238) is associated with a residual fraction ¾ as determined by a sodium hydroxide fusion of the residual material that remained following the extraction procedure (Fig 2a). Plutonium, on the other hand, is distributed primarily among the reducible, organic and nitric-acid extractable fractions in this sediment (Fig 2b). We present selected results from the optimization experiments. A future publication will include a complete interpretation of the results.
Table
2 Summary of the optimized parameter settings
for each fraction.
|
Fraction |
Temperature (°C) |
Concentration (M) |
Duration (hours) |
|
MgCl2 |
25 |
0.4 |
1 |
|
NH4Ac
(pH 5) |
25 |
1.0 |
2.0 |
|
NH2OH-HCl
/ 25% HAc |
70 |
0.1 |
6 |
|
H2O2
/ 0.05M HNO3 |
50 |
pH 2 |
4 |
|
HNO3 |
90 |
4 |
4 |
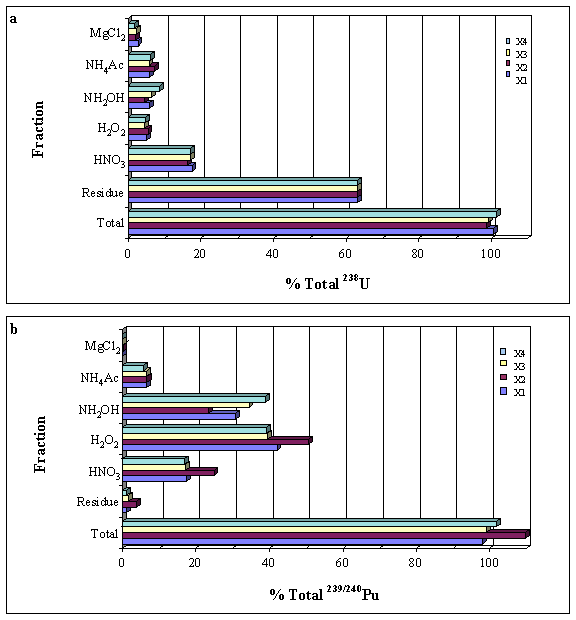
Figure 2. Extraction profiles of U-238 and Pu-239/240 as determined by the NSSEP. The x-axis represents the percent total extracted for four complete runs. The y-axis shows the fractions in order of extraction.
Reference
Box, G. E. P., Hunter W. G. and Hunter, J. S. (1978) Statistics for Experimenters: An Introduction to Design Analysis and Model Building. John Wiley and Sons, New York, NY.
Schultz, M. K., Burnett, W.
C., Inn, K. G. W., Thomas, J. W. L. and Lin, Z. (1996) Partitioning of
radioactive elements in NIST natural-matrix standards. J. Res. Nat. Inst. of Stand. and Technol. 101, 707-715.
Schultz, M. K., Inn, K. G.
W., Lin, Z. C., Burnett, W. C, Smith, G. E., Biegalski, S. R. and Filliben, J.
(1998a) Identification of radionuclide partitioning in soils and sediments:
Determination of the optimum conditions for the exchangeable fraction of the
NIST standard sequential extraction protocol. Appl. Radiat. Isot. 49, 9-11, 1289-1293.
Schultz, M. K., Burnett, W.
C. and Inn, K. G. W. (1998b) Evaluation of a sequential extraction method for
determining actinide fractionation in soils and sediments. J. Environ. Rad. 40, 2, 155-174.
Schultz, M. K., Biegalski,
S. R., Inn, K. G. W., Yu, L., Burnett, Thomas, J. L. W., and Smith, G. E.
(1999) Optimizing the removal of carbon phases in soils and sediments for
sequential chemical extractions by coulometry. J. Environ. Monit., 1, 183-190.
Tessier, A., Campbell, P.
and Bison, M. (1979) Sequential extraction procedure for the speciation of
particulate trace metals. Anal. Chem.
51, 7, 844-850.